Beaked Whales
Updated: June 2020
Beaked whales are a globally distributed family of toothed whales (Ziphiidae or ziphiids), with species found in every ocean. They primarily feed on deep-water squid and fish and are deep divers that are found in deep-sea areas off the continental shelves or on their slopes. Although it is the second-largest cetacean family, with 22 species across 6 genera, it is also likely the least familiar. This is because it is strictly oceanic (i.e. does not spend time close to shore) and has inconspicuous surfacing behaviour. Most species avoid boats and are very difficult to observe, let alone identify at sea. Some species have never been observed at sea and most are best known from strandings.

ABUNDANCE
The family is thought to be abundant throughout its range, although there are few surveys providing current population estimates for the different species.
DISTRIBUTION
Species within the beaked whale family are found in all the oceans of the world.
RELATION TO HUMANS
Only the northern bottlenose whale has been subject to commercial hunt in the North Atlantic in the past. Currently, small scale hunting of this species as a food resource occurs within the North Atlantic.
CONSERVATION AND MANAGEMENT
All beaked whale species are protected throughout the NAMMCO area, except the northern bottlenose whale, which is hunted in Greenland in small numbers and opportunistically taken in the Faroe Islands when animals strand.
The family (Ziphiidae)
The beaked whales make up ¼ of all existing cetacean species, making them the second-largest cetacean family after the delphinids (Johnson et al. 2004, MacLeod et al. 2003). The family represents a monophyletic group (Dalebout et al. 2004), which means that all species in the group descend from a single ancestor. The beaked whale family is comprised of 22 species spread among 6 different genera: Berardius (two species), Hyperoodon (two species), Indopacetus (one species), Mesoplodon (15 species), Tasmacetus (one species) and Ziphius (one species) (IUCN 2018, McLeod 2018, Morin et al. 2017).
In the North Atlantic, there are six resident beaked whale species: Northern bottlenose whale (Hyperoodon ampullatus), Cuvier’s beaked whale (Ziphius cavirostris), Sowerby’s beaked whale (Mesoplodon bidens), True’s beaked whale (Mesolplodon mirus), Blainville’s beaked whale (Mesoplodon densirostris) and Gervais beaked whale (Mesoplodon europeaus).
The northern bottlenose whale is the most northern and most common species. It has been heavily exploited in the past on both sides of the North Atlantic, notably by Norway and Great Britain in the 19th and 20th centuries. You can find more information about this specific species on its species page.
On the remainder of this page about the beaked whale family, we first present some general information on common traits for all beaked whales, followed by some specific information for Ziphius cavirostris (Cuvier’s beaked whale), and three Mesoplodon species (Blainville’s and Sowerby’s and True’s beaked whales). We focus on these four species in particular because they are believed to have their distribution extending into the waters of some NAMMCO member countries (although some only in their extralimital range).
Morphology
Beaked whales have a streamlined spindle-shaped body. They have relatively small pectoral fins that fit into small pigmented depressions/pockets on the side of the animal and a small triangular dorsal fin situated two thirds of the way down the back. The small appendices, together with the body-shape, create the least resistance from the water when the whales perform their deep foraging dives. The species range in size from 3 – 10 m in length, with females being slightly larger than males (Ellis and Mead 2017). Beaked whales have V-shaped throat groves, which are used for suction feeding.
Being a family of toothed whales, all beaked whale species have teeth. However, they only erupt in mature males and are called tusks (except in one single species, which has a complete set of functional teeth in both sexes). The angle and placement of the tusks in the mandible are often so characteristic that they can be used for identifying species. The tusks are believed to be used in male-to-male intra-specific aggressive behaviours, as extensive scarring predominates in mature males in all species.

Three Cuvier’s beaked whales. Note the differentiation in the colouration of the male in the back from the other two animals © Charlie Jackson
Some beaked whale species (e.g. Cuvier’s beaked whale), display an array of colouration and morphological traits that render individuals very different from each other. Meanwhile, other beaked whale species exhibit similarities in morphology and colouration, which makes them difficult to distinguish from each other at sea when encountered. This is particularly true for the genus Mesoplodon (Hemshaw et al. 1997, Heyning 1989). In this group, the shape and size of the beak and the melon, as well as the placement of the tusks in males, is used to distinguish between the species at sea (Aguilar de Soto et al. 2017, Dalebout et al. 2008).
Social interactions
Two different forms of social structure have been recorded in beaked whales, typically linked to species in the different genera. Both structures contain adult females, dependent offspring, and some adult males for which the number depends on group size (MacLeod 2018). These two general social structures are: 1) small groups, typically consisting of one to five individuals, generally with only one mature male. This is usually observed for species in the genera Hyperoodon, Mesoplodon and Ziphius, for which groups larger than 10 individuals are very rare; 2) groups containing five to 20 individuals, usually containing several adult males. This structure has been recorded for species in other genera such as Indopacetus and Berardius and groups aggregations up to 100 individuals have been observed for these genera.
Beaked whale males are thought to display some form of intra-specific aggressive behaviour towards other males after reaching sexual maturity. This is believed because only mature males have extensive scarring and visible tusks in the lower jaw (Pitman 2018, Heyning 1984). However, such behaviour has so far not been confirmed by at-sea observations (Aguilar de Soto et al. 2017).
Feeding and diving
Like other odontocetes, beaked whales use echolocation to orientate themselves and locate their prey in the deep dark ocean water (Madsen et al. 2015). They send a sequence of high-frequency click sounds into the environment. The sounds then bounce off distant objects and the echoes are received back by the animal. These echoes enable beaked whales to judge the distance to prey as well as obstacles. As an echolocating animal gets closer to its target, the rate at which it produces clicks gets faster and faster.
This family has a specialized foraging strategy called suction feeding. By creating a strong pressure-gradient, using their tongue bone (hyoid bone) and v-shaped food groves, they create a vacuum in their mouth which enables them to suck in their prey and swallow it whole (Heyning and Mead 1996).
Beaked whales are generally found in deep water areas off the continental shelves, often along underwater canyons as some species dive down to several thousand meters when foraging. In their search for deep water squid and fish prey, beaked whales perform prolonged dives and 60-minute dives are not uncommon. They also perform shallow dives between long foraging dives to avoid build-up of nitrogen in the lungs and tissues.
Distribution and habitat
Beaked whales are found in all the oceans of the world (MacLeod et al. 2006). Some species have a limited distribution, such as the northern bottlenose whale and Sowerby’s beaked whale, which are only found in the North Atlantic. Other species have a global distribution, such as Cuvier’s beaked whale and Blainville’s beaked whale.
Abundance
The beaked whale family consists of elusive deep-diving species. Their offshore habitat, along with prolonged dive duration, makes abundance estimates for the different species difficult to determine. In addition, Mesoplodon’s are hard to distinguish, due to the morphological similarities between species. Furthermore, the abundance of beaked whales is also underestimated when using the present visual survey methods because of their long diving periods, where the whales are unavailable and thus not observed, as well as due to their inconspicuous behaviour when they surface, which makes them easy to miss (Barlow 1999).
By combining the summer of the 2005 SCANS II survey with the 2007 CODA and Faroese NASS survey (Figure 1), Rogan and colleagues provided a combined abundance estimate for all beaked whale species of 29,154 individuals (95% CI: 17,478 – 48,629) in the North-East Atlantic area in 2017. Of these, 12,900 individuals (CV=0.31) were from the SCANS II-CODA area. These estimates are believed to be underestimates as they have not been corrected for diving or missed animals (Rogan et al. 2017).
The SCANS-III survey was carried out in summer 2016 and covered the waters of the European continental shelf but excluded the waters off Ireland (Figure 2). It also generated a combined abundance estimate for all beaked whale species for this smaller area of 11,394 individuals (95% CI: 4,494 – 28,888) individuals (Hammond et al. 2017). To this estimate it should be added at least 3,142 animals (CV = 0.5), which was the estimate for the Irish waters covered by the ObSERVE survey in the summer of 2016 (Rogan et al. 2018). The observed distribution and estimates are similar for 2007 and 2016 for the equivalent area (Hammond et al. 2017).
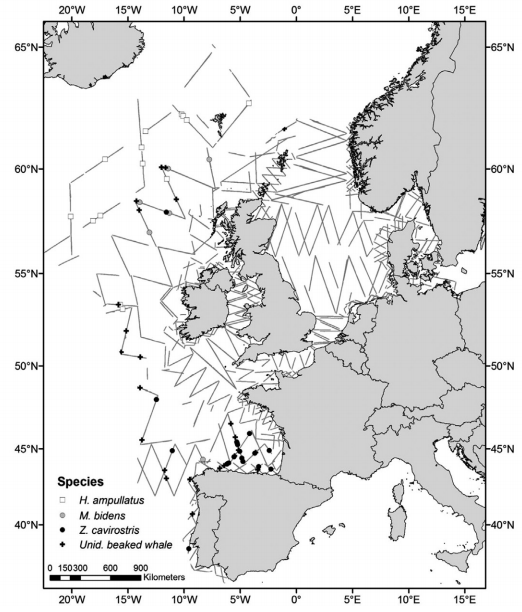
Figure 2: Distribution of survey effort and sightings of beaked whales in the survey area of three dedicated surveys (Rogan et al. 2017)
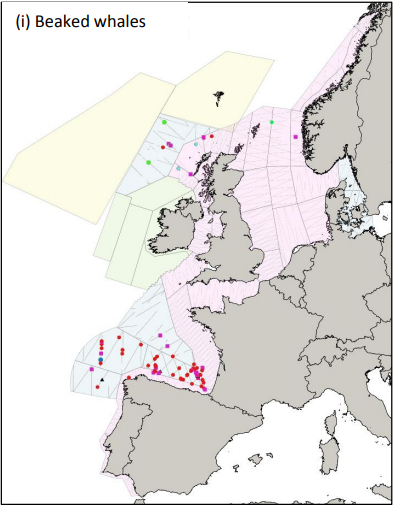
Figure 1: Sightings of beaked whales during the SCANS III sightings surveys in July 2016: Cuvier’s beaked whale – red dot; Gervais beaked whale – blue dot; unidentified beaked whale – pink square; unidentified Mesoplodon – black triangle; Sowerby’s beaked whale – green dot; bottlenose whale – turquoise dot (Hammond et al. 2017).
Research tools
Identifying sex and establishing social structure through genetic techniques is especially helpful when dealing with species that are difficult to observe and little known, like the elusive beaked whales. Indeed, the conservation status of these species also needs to be assessed in order to identify conservation measures if required.
For the whole beaked whale family, it is generally true that females and subadult males are almost impossible to distinguish visually from one another, as they share characteristics such as colouration and lack of apparent dentition. Therefore, in some species, the sex of individuals can only be uncovered analysing biopsy samples using genetic techniques (Pitman 2018).
Using molecular methods, where sequences of the DNA from different beaked whale individuals are compared, researchers are continuously striving to assess the population structure of the different species (Carroll et al. 2016, Dalebout et al. 2005, Dalebout et al. 2006, Hooker et al. 2019, Morin et al. 2017, Thompson et al. 2016).
Conservation and management
All beaked whale species are protected throughout the NAMMCO area, except the northern bottlenose whale, which is still hunted in Greenland.
All beaked whales, except two species (Cuvier’s beaked whale and the southern bottlenose whale), are listed as data deficient on the International Union for Conservation in Nature’s Red List of Threatened Species (IUCN 2018).
The listing for the northern bottlenose whale is “Least Concern” on the Norwegian National Red List and “Data Deficient” on the Icelandic and Greenlandic National Red Lists. All other beaked whale species are listed as “Not Applicable” on the Icelandic National Red List.
Sowerby’s beaked whale is mentioned on the Norwegian National red list where it is listed as “Data Deficient”.
Most species within the family are listed in Appendix II of the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES). This appendix includes species not necessarily threatened with extinction, but in which trade must be controlled in order to avoid utilisation incompatible with their survival.
All beaked whales with a distribution in the North Atlantic are covered by the Agreement on the Conservation of Small Cetaceans of the Baltic, North East Atlantic, Irish and North Seas (ASCOBANS) and the Agreement of the Conservation of Cetaceans of the Black Sea, Mediterranean Sea and Contiguous Atlantic Area (ACCOBAMS).
Relation to humans
Hunt
There is no history of commercial hunting in the North Atlantic for any other beaked whale species than the northern bottlenose whale. For more details, see Hunting and Utilisation on the northern bottlenose whale species page.
Noise / disturbance
In recent years, there has been increasing concern that loud underwater sounds (such as active sonar from military vessels and seismic operations), may be harmful to beaked whales (Malakoff 2002). Beaked whales have been observed ascending rapidly following exposure to sonar during their deep dives (DeRuiter et al. 2013). Necropsies have shown gas bubble lesions in tissues (e.g. portal veins and liver) of stranded specimens, which are similar to the ones found in human divers suffering from decompression sickness following a rapid ascent to the surface (Fernandez et al. 2005, Jepson et al. 2003).
Contaminants and climate change
Marine debris is believed to represent an increasing threat to odontocetes and other marine life in most oceans (Fossi et al. 2018, Laist 1997), and stranded beaked whales have been found with stomachs full of plastics and debris (Gomercic et al. 2006, Puig-Lozano et al. 2018, Secchi and Zarzur 1999).
Global climate change and its impact on the marine environment will affect beaked whales, although exactly how, when and to what extent is still unclear (Learmonth et al. 2006). Changes in prey abundance and distribution due to warming oceans could pose a serious threat, as these species are highly specialised foragers within their deep-water habitat.
Cuvier’s beaked whale
Cuvier’s beaked whale is a cosmopolitan beaked whale species and the most frequently observed. It is one of the larger species in the family with adults reaching up to 7 m in length. The species has a short, poorly defined beak, and a slight concavity on top of its head, which becomes more prominent with age. Colouration varies from dark grey in males to grey-brownish in females, often with a dark eyepatch. The body colour may also appear more reddish-brown in bright sunlight due to diatom infestation. They gradually become paler on the head as they age, which extends further down the dorsal part of the body in males, to an extent where they may appear almost white (Baird et al. 2018). At sea, the whitish concave head and almost beakless “smiling” look, the lack of melon, and the distinctive dark grey to reddish-brown colouration help to differentiate the species from the slightly larger northern bottlenose whales.
The male tusks are positioned at the tip of the lower jaw and scar patterns from intraspecific aggression can be used to identify individuals (Baird et al. 2018, Coomber et al. 2016). Morphological variations have been found between regions, including differences in pigmentation patterns and cranial features (Heyning 1989).
Lifespan
Cuvier’s beaked whales are estimated to live for over 40 years.
Size
They can reach up to 7 metres in length and weigh up to three tonnes.
Reproduction
Calving intervals are not certain, but one observed female had a 6-year interval.
Migration
Cuvier’s beaked whales are known to show some site fidelity, at least in some areas of their distribution.
Feeding
Cuvier’s beaked whale usually preys on many different cephalopod species, but primarily on large species of squid.
Scientific name: Ziphius cavirostris
Faroese: No common name
Greenlandic: As the species is rare in this area, no common name has been given
Icelandic: Gáshnallur
Norwegian: Blekhodenebbhval
Danish: Småhovedet hval
English: Cuvier’s beaked whale, goose-beaked whale
Names
The scientific name of Cuvier’s beaked whale Ziphius cavirostris is New Latin. It is a modification of Greek xiphios swordfish, from xiphos sword and cavirostris derived from the Latin words cavus, meaning “hollow”, and rostrum, meaning “snout”.
Life history
Females reach sexual maturity at around 5.8 m and males at 5.5 m. Reports on the oldest aged individual span from 28 to 30 in females and 36 to 47 years in males (Baird et al. 2018, Ross 2006).
Social interactions
Group sizes are usually small and lone individuals, often males, are not uncommon (Baird et al. 2018, Ross 2006).
Cuvier’s beaked whales in the Ligurian Sea
Sound and communication
Cuvier’s beaked whales are a highly vocal species which produces lots of high-frequency clicks when hunting. However, Cuvier’s beaked whale seems to be completely silent in the upper water column above 500m depth. This is believed to be a predator avoidance behaviour, as it makes them very hard to detect from a distance (Tyack et al. 2006).
Diving and swimming
Cuvier’s beaked whales are known as extreme divers, although behavioural data from this difficult-to-study species are limited.
A study found that Cuvier’s beaked whale, on average, dive to 1888 metres for extended periods of up to 85 minutes (Tyack et al. 2006). In 2011, a tagged Cuvier’s beaked whale dove to 2,992 m, which is the deepest recorded dive by any mammal. The longest dive recorded during the study lasted for 137,5 minutes (Schorr et al. 2014).
Cuvier’s beaked whales swimming near Savona in the Ligurian Sea
Food and feeding
Cuvier’s beaked whales usually prey on many different cephalopod species, but primarily on large specimens of squid (MacLeod et al. 2003). They find their prey in the deep ocean waters using echolocation; a sequence of high-frequency click sounds that are sent into the environment. They then use suction feeding to catch their prey (Heyning and Mead 1996).
Predators
This species has no known predators. It may be presumed that orcas could be a predator, however, predation is made difficult by Cuvier’s beaked whales emitting no sounds in the top 500 m of water depth.
Strandings
Strandings of dead Cuvier’s beaked whales have been associated with naval exercises all over the globe. They are the species most often stranded in association with Mid-Frequency Active (MFA) sonar use, a relationship that remains poorly understood (D’Amico et al 2009) – for more information see section on noise.
In 2017, a Cuvier’s beaked whale stranded on Vindenes, Fjell, Sotra Island, in the Hordaland county of the Vestlandet region of the Norwegian coast. The whale was an adult female, 6.25 m long and weighed around 2 tonnes (Bachara and Øien 2017). This was only the second recorded Cuvier’s beaked whale to have stranded in Norway. The cause of death for this individual was not established.
Distribution and habitat
Distribution
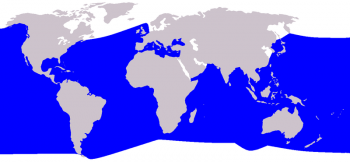
Cuvier’s beaked whale distribution ©Pcb(talk) after Vardion
The Cuvier’s beaked whale has the largest distributional range of all ziphiids. It is found in offshore waters of all oceans, from tropical to polar regions oceans. It is not found in very high-latitude polar regions or shallow water areas. These whales are common in the North-East Atlantic but are absent from the Baltic sea. It is also the only species of beaked whale regularly found in the Mediterranean Sea.
Movements and migration
Despite their extensive distribution range, studies show long-term site fidelity (philopatry) and local adaptations in Cuvier’s beaked whales (McSweeney et al. 2007, Hoelzel 1998). The global assessment of Cuvier’s beaked whales’ genetic diversity suggests that there is probably little movement of the whales among different ocean basins and that there may even be a distinct subpopulation in the Mediterranean Sea (Dalebout et al. 2005).
Stocks and abundance
Worldwide
Cuvier’s beaked whales are undoubtedly among the most common and abundant of all the beaked whales and worldwide abundance is likely to be well over 100,000 (Taylor et al. 2008).
North Atlantic
By combining the data from three surveys (2005 SCANS-II, and 2007 CODA and Faroese area of T-NASS) Rogan et al. (2017) suggest an abundance of 2,286 Cuvier’s beaked whales (CI: 95%: 942–5,552) for the area. This abundance estimate is the first-ever for these waters (see section on abundance in the general introduction of beaked whales for an illustration) and gives researchers a better understanding of the abundance of this deep-diving species in the North-East Atlantic.
Specific management measures
In 2004, the Parties to the UNEP CMS Agreement on the Conservation of Cetaceans of the Black Sea, Mediterranean Sea and Contiguous Atlantic Area (ACCOBAMS) adopted a resolution recommending avoiding activities generating high-intensity noise in areas with a high abundance of Cuvier’s beaked whales.
Human impacts
Human removals: hunt & by-catch
The species has not been hunted in the North Atlantic, although it has been taken opportunistically in other direct fisheries in the Caribbean islands, as well as in the oceans around Peru, Chile, Indonesia, Japan and Sri Lanka (Taylor et al. 2008).
Cases of Cuvier’s beaked whale being by-caught have been reported in different fisheries, including the Italian swordfish fishery (Taylor et al. 2008), although by-catch in the North-East Atlantic seems very low, if not absent.
Noise
Cuvier’s beaked whales have proved to be extremely sensitive to noise disturbance (Schorr et al. 2014). Strandings of Cuvier’s beaked whales, either single or en masse, were very rare until the 1950s. However, as the use of sonar was introduced in vessels (industrial, research and military), the prevalence of stranding events has risen greatly (Ketten 2014, Bernaldo de Quiro’s et al. 2019).
Cuvier’s beaked whale is the dominant species in mass stranding events and several mass strandings of this species have occurred in the North Atlantic in recent decades. This includes events in the Mediterranean Sea during 1996 (Frantzis 1998), the Madeira Islands in 2000 (Frietas 2004), and the Canary Islands in 2002 (Jepson et al. 2003). These mass strandings have been related to behaviour to avoid sonar disturbance (Aguilar de Soto et al. 2016, New et al. 2013, Cox et al. 2006).
Within the North-East Atlantic, three mass stranding events have occurred around the British Isles in recent decades. In 2008, 18 Cuvier’s beaked whales stranded along the coastline over seven months (Dolman et al. 2010). In December 2014/January 2015 five stranding events were documented (Brownlow et al. 2015). The latest event happened between July and October of 2018 where 83 Cuvier’s beaked whales stranded along the coastline of the Isles. During the latest mass stranding event, there was also an increase in the observed number of live or dead strandings of beaked whales (15 individuals) reported in Iceland, which was believed to be connected to the strandings along the British and Irish coastlines. This lead to a total of 102 Cuvier’s beaked whale strandings in 2018 (Brownlow et al. 2019).
Did you know?
Cuvier’s beaked whale has the all-time deep-diving record for any mammal on the planet. An individual was confirmed to dive to 2992 meters for 137.5 minutes!
Mesoplodon

Male Blainville’s beaked whale © Oregon State University
The Mesoplodon beaked whales include 14 species that are all small to medium-sized. They are rarely positively identified at sea and their life history, behaviour and ecology is very poorly understood. Some have a wide distribution over several ocean basins, while others have restricted ranges. Lifespans are often unknown, but are likely relatively long, i.e. 20+ years. Females are sexually mature at 8-10 years of age. The breeding season is believed to be from late winter to spring. Females give birth to a single calf (Pitman, 2018). Gestation periods are unknown but thought to be 12 months in most species.
Blainville’s beaked whale

This species was first described in 1817 by the French zoologist Henri de Blainville based on the finding of a piece of jawbone. The jawbone is denser than elephant ivory, making it the densest bone ever examined. The scientific name of the species relates to this particularity, with densirostris meaning “dense beak” in Latin. The species has a very characteristic bulged lower jaw. The arch of the lower jaw increases with age and becomes very prominent in males. The single pair of massive teeth are placed at the apex of the arch and are angled forward. With its global distribution in all tropical to temperate waters, it is the widest-ranging Mesoplodon species. Very little is known of this species throughout most of its range, but it has been well studied the northeastern Bahamas, where it comes close to shore.
Lifespan
Unknown – see the general introduction to Mesoplodon.
Size
Up to 5 metres in length and one tonne in weight.
Reproduction
Females are believed to be mature at about 9 years of age and give birth to a single calf.
Migration
No migration has been described but long-term site fidelity (philopatry) and local adaptations have been documented.
Feeding
Mainly various deep-sea squids species, but also occasionally fishes and crustaceans.
Names
The name Mesoplodon roughly translates to armed with a tooth in the centre of the jaw in greek, mes-mesos = middle, -opl-, hoplon = tool, weapon, and -odon, odous = tooth. The presence of this tooth is a well known characteristic for all beaked whales.

Scientific name: Mesoplodon densirostris
Faroese: No common name
Greenlandic: As the species is rare in this area, no common name has been given
Icelandic: Króksnjáldri
Norwegian: Blainvilles spisshval
Danish: Blainvilles næbhval
English: Blainville’s beaked whale, dense-beaked whale
Morphology

Mother and calf pair of Blainville’s beaked whales © Matthew Grammatico
Blainville’s beaked whales reach 4-6 m in length and weigh 800-1000 kg. They have a large dolphin-like body with a flattish forehead and an indistinct melon. Like other beaked whales, males have tusks and extensive scarring. The strongly arched lower jaw and the protruding tusk gives adult males a very unique profile. The tusk-like teeth are also sometimes covered with barnacles. This species has the densest bone ever described and is often referred to as the ‘dense-beaked whale’ (Ellis and Mead 2017).
Life history
Females are believed to be sexually mature at around 9 years of age (Pitman 2018). Newborn calves are about 1.80 to 2.5 m long and weigh about 60 kg.
Behaviour
Individual males tend to be found alone or in small groups, while females with calves seem to prefer to associate in larger groups, consisting of multiple adult females. Larger groups typically only have one male present. This, combined with the scarring in males, is suggestive of some form of female defence polygyny within this species (Hooker et al. 2019). Studies of association patterns suggest that male-female associations might last for at least a year (Dunn 2015, Hooker et al. 2019).
Diving and feeding
The dives performed by Blainville’s beaked whales usually last between 20 – 45 minutes and can commonly reach depths of at least 500 to 1000 m. However dives of over 54 minutes and up to 1400 m have also been recorded (Jeffersen et al. 2008). While diving, they use the strategy of suction feeding to prey on small fish and cephalopods (e.g. squid) in deep water.
Distribution and habitat
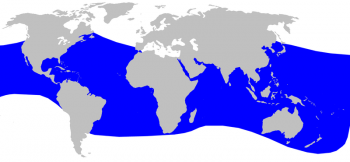
Blainville’s beaked whale distribution © Alessio Marrucci
The Blainsville’s beaked whale has one of the widest distributional ranges, although it extends less towards higher latitudes than the Cuvier’s beaked whale. It is found in tropical and temperate oceans in both hemispheres and in continental slope waters of intermediate depth (between 200 and 1,000 m). It can also occur in coastal waters around oceanic islands. The species occurs in many enclosed seas with deep waters, but there are only rare records from the Mediterranean and the species is therefore probably a vagrant species there (Taylor et al. 2008).
Very little is known about this species, except off the northeastern Bahamas where the animals are particularly well documented through long-term studies. These studies have been conducted since the 1990s and offer a wealth of information on the ecology of that population (Claridge 2013).
Abundance
Overall, the species appears to be fairly common in most tropical seas, although it is not considered to be common throughout the whole distribution area per se (Ellis and Mead 2017). It is one of the most common of all the species of Mesoplodon. Abundance estimates only exist for a few areas and no estimate is currently available in the NAMMCO area.
Threats
Blainville’s beaked whales have been and are opportunistically taken in artisanal fisheries in various areas, but likely not in the North Atlantic.
As for other beaked whales, there is an increasing concern that loud underwater sounds, such as active sonar and seismic operations, may be harmful and the use of active sonar from military vessels has been implicated in mass strandings of Blainville’s beaked whales (e.g. Jepson et al. 2003, Taylor et al. 2008).
This species will face many of the same threats from human activities described for beaked whales in general. For example, some stranded specimens have been found with plastic in their stomachs indicating that marine pollution can pose a potential threat.
Conservation and management
See the Conservation and Management section under general information.
Did you know?
The jawbone of Blainville’s beaked whale is the densest bone ever recorded. It is even denser than ivory. It is the bone density that gave this species the name densirostris, which translates to “dense bone”.
Mesoplodon

Sketch of Sowerby’s beaked whale male © Thorburn, Archibald
The Mesoplodon beaked whales include 14 species of beaked whales all small to medium-sized and rather unknown. They are rarely positively identified at sea and their life history, behaviour and ecology is very poorly known. Some have a wide distribution over several ocean basins, some have restricted ranges. Lifespans are often unknown, but likely relatively long of 20+ years. Females are sexually mature at 8-10 years of age. Their breeding season is believed to be from late winter to spring. Females give birth to a single calf (Pitman, 2018). Gestation periods are unknown but thought to be 12 months in most species.

Sowerby’s beaked whale
The Sowerby’s beaked whale was the first mesoplodont whale to be described, in 1804, but it has still primarily been described on the basis of strandings. Sowerby’s beaked whale is a species found exclusively in the North Atlantic ocean. This species inhabits the deep waters beyond the continental shelf in the temperate and subarctic regions. They have a spindle-shaped body and a long slender beak resembling that of dolphins. They can reach 5 m in length. The pectoral fins are short and narrow, fitting into “pockets” and the dorsal fin is small, triangular and placed two thirds of the way down the back. The colouration is blueish-grey with speckles of white and there is typically extensive scarring patterns on males.
Size
Sowerby’s beaked whales can reach a length of 5.5 m and weigh between 1 – 1.3 tonnes.
Lifespan
The lifespan of this species is unknown.
Reproduction
Females are believed to mature at about 10 years of age, however further information on reproduction is unknown.
Migration
No migration patterns have yet been described.
Feeding
Their primary prey is squid, but they also eat mesopelagic and bottom-dwelling fish species.
Names
The name Mesoplodon roughly translates to armed with a tooth in the centre of the jaw in greek, mes-mesos = middle, -opl-, hoplon = tool, weapon, and -odon, odous = tooth. The presence of this tooth is a well known characteristic for all beaked whales.

Scientific Name: Mesoplodon bidens
Faroese: Nehvalur
Greenlandic: Rare in this area, no common name given
Icelandic: Norðursnjáldri
Norwegian: Nordspisshval
Danish: Næbhval
English: Sowerby’s beaked whale, North Atlantic beaked whale
General characteristics
Sowerby’s beaked whales have a torpedo body shape, which is classic for the Mesoplodon genus. This species was first described in 1804 based on the finding of a jawbone (Ellis and Mead 2017).
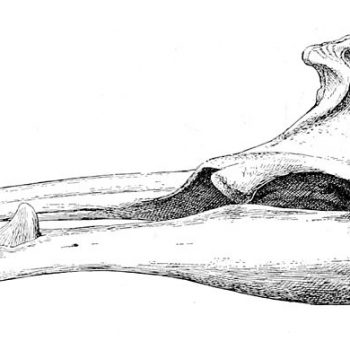
Sketch of the skull of an adult male Sowerby’s beaked whale with the erupted tusk clearly visible © Royal Natural History
They are characterised by the single pair of teeth protruding midway along the jawline. The small triangular dorsal fin is placed two thirds of the way down the back and the species has narrow pectoral fins. They are bluish-grey in colour with light brown colouration on the head and beak and countershading on the ventral side (i.e. on the belly). As for all other beaked whale species, adult individuals – predominantly males – have extensive scarring patterns. Apart from dentition differences, there is little sexual dimorphism in this species (Mead 1989).
Life history and ecology
Life history
Adult individuals can reach between 5 – 5.5 m in length and can weigh up to 1.3 tonnes. Virtually nothing is known about the life hisotyr of the Sowerby’s beaked whale. Offspring/young are around 2.4-2.7 meters at birth and weigh about 185kg, i.e., about 40-50% of the mother’s size.
Social interactions
This species is usually found in pairs or in pods up to ten individuals of mixed age and sex.
Diving and swimming
Sowerby’s beaked whales have been observed bringing their entire beak out of the water when they surface. This is often done at a steep 45○ angle (Hooker and Baird 1999, Pitman 2018). Sowerby’s beaked whale usually exhibits little surface behaviour, but tail-slapping, breaching and spy-hopping have been observed (Hooker and Baird 1999).
Sowerby’s beaked whale, like most other Mesoplodon species, perform deep dives. Foraging dives of up to 28 minutes have been recorded, with whales only staying at the surface for a few minutes in between (Culik 2003).
Food and Feeding
Like other beaked whales, Sowerby’s beaked whales use suction feeding. Their prey mostly consists of midwater fish, deep-water squid, and molluscs (Pereira et al. 2011, MacLeod et al. 2003, Ostrom et al. 1993).
Predators
Orcas and large sharks are likely predating on this species.
Distribution and habitat
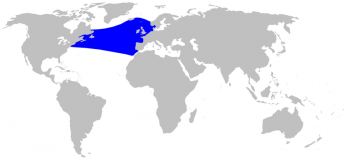
Sowerby’s beaked whale distribution © Alessio Govi
Distribution
The Sowerby’s beaked whale is found exclusively in the North Atlantic and inhabits temperate to subarctic Atlantic waters, from the American/Canadian east coast (up to Newfoundland and Labrador) to Iceland and the Norwegian Sea, and south to Madeira and Morocco (Cosewic 2006). Sowerby’s beaked whale is the most northernly distributed Mesoplodon species with a southern limit at around 30°N. (Macleod 2000), although its presence is uncertain in Greenlandic waters (Taylor et al. 2008).
Habitat
Even though the species is well described from strandings, little knowledge is available about their habitat as they are seldom observed in the wild (Culik 2003). They have been mostly observed offshore in the deep waters beyond the continental shelf in the North Atlantic ocean (MacLeod 2000).
Did you know?
Of all the Atlantic species of Mesoplodon this is the one with the most northerly distribution, with sightings as far as 71°N in the Norwegian Sea (Carlstrøm et al. 1997)
Movements and migration
There is little knowledge regarding the migration pattern of Sowerby’s beaked whales. It is unknown if individuals move between the east and west Atlantic (Cosewic 2006).
Abundance and trends
Their overall abundance and stock structure is unknown, but Sowerby’s beaked whales are not believed to be uncommon and are one of the most common Mesoplodon species in some areas of the North Atlantic.
The first-ever overall abundance of Sowerby’s beaked whales for the oceanic and shelf waters of the North-East Atlantic was obtained by combining the data from three surveys (2005 SCANS-II, 2007 CODA, and Faroese T-NASS, see Figure 2 in the general introduction). It generated an abundance of 3,518 (CI 95%: 1,570–7,883) for the surveyed area (Rogan et al. 2017).
The habitat and abundance of Sowerby’s beaked whale are overall too poorly known to speculate on trends (Taylor et al. 2008).
Human impacts
There is little specific information on the threats to this species (Reeves et al. 2003).
Human removals: hunt & by-catch
Sowerby’s beaked whales were incidentally killed by whalers in Newfoundland, Iceland, and in the Barents Sea. A few entanglements in fishing gear (e.g., driftnets) have been documented. Waring et al. (2001) reported that from 1989-1998, the observed by-catch in pelagic drift gillnets along the US East Coast included 24 Sowerby’s beaked whales.

Drawing of Sowerby’s beaked whale © Biodiversity heritage library
Strandings
This is the most common Mesoplodon species recorded in stranding events. On the Irish coast, there have been ten reported strandings of Sowerby’s beaked whales since 2004 (McGovern et al. 2016). Although the cause of death is often not established, in 2018 a Sowerby’s beaked whale was necropsied and the cause of death was determined to be boat/ship strike (Brownlow et al. 2018).
Noise/disturbance
Since they hunt at great depths, Sowerby’s beaked whales (as all other beaked whales), are vulnerable to anthropogenic noise such as military mid-range sonar and seismic surveys. Individuals of other Mesoplodon species stranded in spatial and temporal proximity to military exercises have displayed pathology matching gas-bubble disease, which led to the hypothesis of abnormal diving behaviour in these specimens due to noise disturbance (Cox et al. 2006, Tyack et al. 2006, COSEWIC 2008).
Climate change
Climate change will have different impacts on different cetacean species according to their distribution, habitat and prey. As Sowerby’s beaked whale is a temperate/subarctic species, depending on a specific group of deep-sea prey species, the consequences for this species are highly correlated to the consequences for the squid they feed upon.
Conservation and management
The species is designated as “Special Concern” by the Committee on the Status of Endangered Wildlife in Canada (COSEWIC).
For more on the conservation and management of beaked whales in the North-East Atlantic, see the section on Conservation and Management under general information.
Did you know?
The species name bidens is derived from the latin words for two(bi) and teeth(dens), due to the observation of their two visible teeth in the lower jaw.
Mesoplodon
Mesoplodon includes 14 species of beaked whales, which are all small to medium-sized. They are rarely positively identified at sea and their life history, behaviour and ecology is very poorly known. Some have a wide distribution over several ocean basins, while others have restricted ranges. Lifespans are often unknown, but are likely relatively long (20+ years). Females are sexually mature at 8-10 years of age. Their breeding season is from late winter to spring. Females give birth to a single calf (Pitman, 2018). Gestation periods are unknown but believed to be 12 months in most species.

True’s beaked whale
True’s beaked whale is best characterised by its split anti-tropical distribution. The species is found in the North Atlantic between Europe and the USA, from the Atlantic coast of Brazil to South Africa, and in the South Indian Ocean, with no sightings in the tropical waters in-between. True’s beaked whales inhabit the deep waters beyond the continental shelf like all the other beaked whale species. They have a spindle-shaped body, a short prominent beak, and reach around 5 m in length. Their colouration is grey with dark eye patches and a lighter colouration on the head and ventral side (underside). These whales forage on squid and occasionally fish in the deep oceanic water off the continental shelf (Pitman 2018).
Size
True’s beaked whales can reach a length of 5 m and weigh around 1.4 tonnes for both sexes.
Lifespan
The lifespan is unknown.
Reproduction
Unknown.
Migration and Movements
No migration patterns have so far been described.
Feeding
Their primary prey is squid and likely they also occasionally eat mesopelagic and bottom-dwelling fish species.
Names
The name Mesoplodon roughly translates to armed with a tooth in the centre of the jaw in greek, mes-mesos = middle, -opl-, hoplon = tool, weapon, and -odon, odous = tooth. The presence of this tooth is a well known characteristic for all beaked whales.
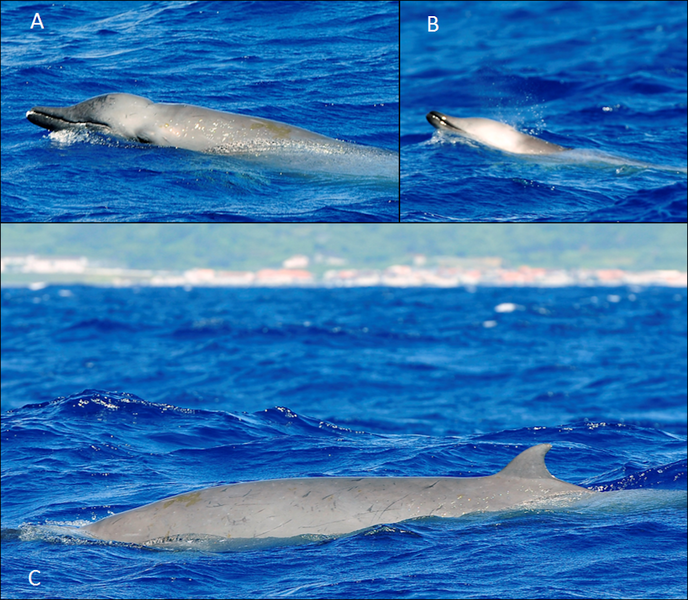
Scientific Name: Mesoplodon mirus
Faroese: No common name
Greenlandic: Not considered resident here and has not been given a common name here.
Icelandic: Fagursnjáldri
Norwegian: Trues spisshval
Danish: Trues næbhval
English: True’s beaked whale,
Morphology
True’s beaked whale is characterised by a slightly bulbous forehead (melon), a prominent short beak, and teeth (measuring around 5cm) located at the tip of the lower jaw and angling forward (Pitman 2018). The scarring pattern of male True’s beaked whales is predominantly parallel and linear, with small gaps between adjacent scars. The pectoral fins are short and the dorsal fin is small, triangular and placed two thirds of the way down the back.
Regional differences
True’s beaked whales are greyish in colouration and usually have a darker patch around the eye and lighter colouration on the head and ventral side. There is some regional variety, both within and between the populations in the northern and southern hemispheres. True’s beaked whales in the North Atlantic are described as almost uniformly greyish in colouration. Individuals have been observed with both a dark eye mark and a dark blaze from behind the blowhole to past the dorsal fin. Some animals may exhibit a pale colouration on the ventral side (the underside), which sometimes extends to the lower jaw. Some animals may also have a pale blaze on their melon (Weir et al. 2004, Jefferson et al. 2015). In contrast, observations in the southern hemisphere have shown a female with more whitish colouration on the dorsal side, extending from the dorsal fin to the tail peduncle (Aguilar de Soto et al. 2017).
The differentiation in colouration has led researchers to question whether the animals in their respective hemispheres are genetically isolated and might represent different species (Dalebout et al. 2007, Macleod et al. 2006). Indeed, some degree of a phylogeographic pattern has been detected in the genetic structure (i.e. considering the geographic distribution of individuals in light of population genetics) (Aguilar de Soto et al. 2017). With the ecology and population structure of True’s beaked whale being virtually unknown, genetic studies are an important contribution to research on this species. The scarcity of information on the species is re-enforced by the rarity of live sightings. The first-ever video recording of this species was in the Azores in 2017 (Aguilar de Soto et al. 2017) and the first photos were taken in the Bay of Biscay area in 2017 (Robbins et al. 2019).
This video shows the first-ever recording of True’s beaked whale in the Azores in 2017 (Aguilar de Soto et al. 2017). Video credit: Roland Edler.
Life history and ecology
Life history
Adult individuals reach around 5 m in length and weigh up to 1.4 tonnes. There is virtually no knowledge about the life history of True’s beaked whale, but offspring/young are around 2-2.5 m at birth i.e., about 40-50% of the mother size.
Social interactions
This species is usually found in pairs or small pods. Males are believed to interact in male to male aggressive behaviour, as adult individuals exhibit a pattern of parallel linear scarring.
Diving and swimming
True’s beaked whale usually exhibits little surface behaviour, although repeated breaching has been observed in the Canary Islands (Aguilar de Soto et al. 2017). This species, like most other Mesoplodon species, perform deep foraging dives.
Food and feeding
Like other beaked whales, True’s beaked whales use suction feeding. Their prey is predominantly deep-water squid of the Loligo spp (MacLeod 2005).
Stable isotope analysis has found that this species feeds at a similar trophic level to other Mesoplodon species with which it is sympatric (two related species or populations coexisting in the same geographic area), but at a lower trophic level than Cuvier’s beaked whale and the northern bottlenose whale. This suggests that True’s beaked whale feeds on smaller prey than the two latter species (MacLeod 2005).
Predators
Orcas and large sharks are likely predating on this species.
Distribution and habitat
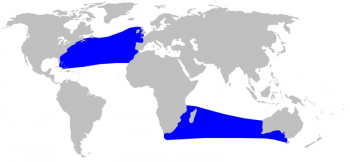
True’s beaked whale distribution © Alessio Marrucci
Distribution
Global
The distribution range for this species is unique among Ziphiids as they are found in two distinct zones. There is one northern distribution in the temperate part of the Atlantic Ocean and a southern distribution in the temperate South Atlantic (strandings in Brazil and South Africa) and the South Indian Ocean, with no confirmed sightings in between. Until 1969, this species had only been confirmed in the North Atlantic, however the stranding of a pregnant female with a calf on the coast of Port Elizabeth, South Africa confirmed the existence of a breeding population in the southern hemisphere as well (Ross 1969).
North Atlantic
In the North Atlantic, the distribution of True’s beaked whales reaches from eastern North America (Nova Scotia to Florida) to Bermuda and in Europe, from the British Isles to the Canary Islands, the Bay of Biscay, and the Azores.
Habitat
The species is mostly described from strandings and little knowledge is available about their habitat as they are seldom observed in the wild. When observed they are, however, found offshore in the deep waters beyond the continental shelf.
Did you know?
The species received its common name from Frederick W. True, a curator at the Smithsonian Institution, who described the species from a single specimen that stranded on a beach in North Carolina.
Movements and Migration

Breaching Trues beaked whales (Aguilar de Soto et al. 2017).
No information is available about True’s beaked whales’ migration pattern.
Abundance and Trends
There are no estimates of abundance for this species, neither globally nor regionally. The species is, however, not considered rare in the North Atlantic.
Human impacts
There is little specific information on the threats of this species, but it is generally thought to be the same as in other beaked whales (Reeves et al. 2003). See general threats on beaked whales
Human removals: Hunt / By-catch
No direct catches of the species have been reported. Entanglement in fishing gear, especially gillnets in deep water (e.g., for billfish and tuna), is probably the most significant threat (Taylor et al. 2008).
Noise/disturbance
See the general introduction to beaked whales
Strandings
For the North Atlantic, most strandings have occurred in the southern temperate water. A 3.7 m long subadult male True’s beaked whale with unerupted teeth stranded (dead) in the Azores in 2004, (Silva et al. 2014). An adult male stranded on the island of Lanzarote (Vonk and Martin 1988) in the Canary Islands. Both animals were identified as True’s beaked whales by their general morphology. Strandings of True’s beaked whales have also been reported off the British Isles, most recently with two strandings on the Irish Coast in 2018 (BBC 2018).
Climate change
Climate change will have different effects on cetacean species in accordance with their distribution and habitat. True’s beaked whales depend on deep-sea cephalopod prey species, and any impact on this specific group of species, either directly, e.g. shift in distribution, or indirectly, e.g. habitat alteration, will have consequences for the True’s beaked whales.
Conservation and Management
For more information see the section on Conservation and Management under general information.
Did you know?
Frederick W. True gave the True’s beaked whale the species name mirus which is Latin for wonderful or amazing, which gave this species the alternative common name wonderful beaked whale.
General introduction
Aguilar de Soto, N., Martin, V., Silva, M., Edler, R., Reyes, C., Carrillo, M., … and Carroll E. (2017). True’s beaked whale (Mesoplodon mirus) in Macaronesia. Peer Journal, 5, e3059. https://doi.org/10.7717/peerj.3059
Barlow, J. (1999). Trackline detection probability for long-diving whales. In J. L. Laake, D. G. Robertson, Steven C. Amstrup (eds.), Marine Mammal Survey and Assessment Methods, 209–221. Rotterdam: Balkema.
Carroll, E. L., Reyes, C., Gaggiotti, O. E., Olsen, M. T., Maaholm, D. J. and Rosso, M. (2016). Pilot study to assess the utility of ddRAD sequencing in identifying species-specific and shared SNPs among Blainville’s (Mesoplodon densirostris) and Cuvier’s (Ziphius cavirostris) beaked whales. In Report SC/66b/DNA/03 Presented to the Scientific Committee. London: International Whaling Commission.
Dalebout, M., Baker, C. S., Mead, J. G., Cockcroft, V. G. and Yamada, T. K. (2004). A comprehensive and validated molecular taxonomy of beaked whales, family Ziphiidae. Journal of Heredity, 95(6), 459-473. https://doi.org/10.1093/jhered/esh054
Dalebout, M. L., Robertson, K. M., Frantzis, A., Engelhaupt, D., Mignucci-Giannoni, A. A., Rosario-Delestre, R. J. and Baker, C. S. (2005). Worldwide structure of mtDNA diversity among Cuvier’s beaked whales (Ziphius cavirostris): Implications for threatened populations. Molecular Ecology, 14, 3353-3371. https://doi.org/10.1111/j.1365-294X.2005.02676.x
Dalebout, M., Ruzzante, D. E., Whitehead, H. and Øien, N.I. (2006). Nuclear and mitochondrial markers reveal distinctiveness of a small population of bottlenose whales (Hyperoodon ampullatus) in the western North Atlantic. Molecular Ecology, 15, 3115-3129. https://doi.org/10.1111/j.1365-294X.2006.03004.x
Dalebout, M., Steel, D. and Baker, C. S. (2008). Phylogeny of the beaked whale genus Mesoplodon (Ziphiidae: Cetacea) revealed by nuclear introns: Implications for the evolution of male tusks. Systematic Biology, 57(6), 857-875. https://doi.org/10.1080/10635150802559257
DeRuiter, S. L., Southall, B. L., Calambokidis, J., Zimmer, W. M. X., Sadykova, D. and Falcone, E. A., (2013). First direct measurements of behavioral responses by Cuvier’s beaked whales to mid-frequency active sonar. Biology Letters, 9. https://doi.org/10.1098/rsbl.2013.0223
Ellis, R. and Mead, J.G. (2017). Beaked Whales: A Complete Guide to Their Biology and Conservation. Baltimore, US: Johns Hopkins University Press. https://doi.org/10.1353/book.52552
Fernandez, A., Edwards, J. F., Rodríguez, F., Espinosa de Los Monteros, A., Herráez, P., Castro, … and Arbelo, M. (2005) “Gas and fat embolic syndrome” involving a mass stranding of beaked whales (Family Ziphiidae) exposed to anthropogenic sonar signals. Veterinary Pathology, 42, 446-457. https://doi.org/10.1354/vp.42-4-446
Fossi, M. C., Panti, C., Baini, M. and Lavers, J. L. (2018). A review of plastic-associated pressures: Cetaceans of the Mediterranean Sea and eastern Australian Shearwaters as case studies. Frontiers in Marine Science, 5, 173. https://doi.org/10.3389/fmars.2018.00173
Gomerčić, H., Gomerčić, M. D., Gomerčić, T., Lucić, H., Dalebout, M., Galov, T., … and Huber, D. (2006). Biological aspects of Cuvier’s beaked whale (Ziphius cavirostris) recorded in the Croatian part of the Adriatic Sea. European Journal of Wildlife Research, 52, 182–187. https://doi.org/10.1007/s10344-006-0032-8
Hemshaw, M. D., LeDuc, R. G. and Chivers, S. J. (1997). Identifying beaked whales (family Ziphiidae) using mtDNA sequences. Marine Mammal Science, 13(3), 487-495. https://doi.org/10.1111/j.1748-7692.1997.tb00656.x
Heyning, J. E. (1984). Functional morphology involved in intraspecific fighting of the beaked whale, Mesoplodon carlhubbsi. Canadian Journal of Zoology, 62, 1645–1654. https://doi.org/10.1139/z84-239
Heyning, J. E. (1989). Cuvier’s beaked whale Ziphius cavirostris G. Cuvier, 1823. In S. H. Ridgway and R. J. Harrison (eds.), Handbook of Marine Mammals, volume 4: River dolphins and the larger toothed whales, 289-308. London, UK:Academic Press.
Heyning, J. E. and Mead, J. G. (1996). Suction Feeding in Beaked Whales: Morphological and Observational Evidence. Natural History Museum of Los Angeles County, 464, 1-12.
Hammond, P. S., Lacey, C., Gilles, A., Viquerat, S., Börjesson, P., Herr, … Øien, N. (2017). Estimates of cetacean abundance in European Atlantic waters in summer 2016 from the SCANS-III aerial and shipboard surveys. University of St. Andrews. Available at: St.Andrews University
Hooker, S. K., Aguilar de Soto, N., Baird, R.W., Carroll, E. L., Claridge, D., Feyrer, L., …, Whitehead H. (2019). Future directions in research on beaked whales. Frontiers in Marine Science, 5(514). https://doi.org/10.3389/fmars.2018.00514
Jepson, P. D., Arebelo, M., Deaville, R., Patterson, I. A. P., Castro, P., Baker, … and Fernandez, A. (2003). Gas-bubble lesions in stranded cetaceans. Nature, 425, 575-576. https://doi.org/10.1038/425575a
Johnson M., Madsen P.T., Zimmer W.M.X., Aguilar de Soto N. and Tyack P.L. (2004). Beaked whales echolocate on prey. Proceedings of the Royal Society London B, 271, 383-386. https://doi.org/10.1098/rsbl.2004.0208
Laist, D. W. (1997). Impacts of marine debris: entanglement of marine life in marine debris including a comprehensive list of species with entanglement and ingestion records. In. J. M Coe and D. B. Rogers (eds.), Marine debris: sources, impacts and solutions, 99–139. New York, NY: Springer. https://doi.org/10.1007/978-1-4613-8486-1_10
MacLeod, C. D., Santos, M. B. and Pierce, G. J. (2003). Review of data on diets of beaked whales: evidence of niche separation and geographic segregation. Journal of Marine Biology, 83, 651-665. https://doi.org/10.1017/S0025315403007616h
Macleod, C. D., Perrin, W. F., Pitman, R. L., Barlow, J., Balance, L., D’amico, A., Gerrodette, T., Joyce, G., Mullin, K. D., Palka, D. L. and Waring, G. T. (2006). Known and inferred distributions of beaked whale species (Ziphiidae: Cetacea). Journal of Cetacean Research and Management, 7(3), 271-286. https://archive.iwc.int/pages/search.php?search=%21collection15&k=
MacLeod, C.D., (2018). Beaked whales, overview. In B. Würsig, J. G. M. Thewissen and K. M. Kovacs (eds.), Encyclopedia of Marine Mammals (third edition), 80-83. London, UK: Elsevier. https://doi.org/10.1016/B978-0-12-804327-1.00062-5
Madsen, P. T., Aguilar de Soto, A., Tyack, P. L. and Johnson, M. (2015) Beaked whales. Current Biology 24(16), 728-730. https://doi.org/10.1016/j.cub.2014.06.041
Malakoff, D. (2002). Suit ties whale deaths to research cruise. Science, 298(5594), 722-723. https://doi.org/10.1126/science.298.5594.722
Morin P.A., Baker C.S., Brewer R.S., Burdin A.M., Dalebout M.L., Dines J.P., … and Wade P.R. (2017). Genetic structure of the beaked whale genus Berardius in the North Pacific, with genetic evidence for a new species. Marine Mammal Science, 33(1), 96-111. https://doi.org/10.1111/mms.12345
Pitman, R. (2018). Mesoplodon Beaked Whales. In B. Würsig, J. G. M. Thewissen and K. M. Kovacs. Encyclopedia of Marine Mammals (third edition), 595 – 601. London, UK: Elsevier. https://doi.org/10.1016/B978-0-12-804327-1.00172-2
Puig-Lozano R., Bernaldo de Quirós Y., Díaz-Delgado J., García-Álvarez N., Sierra E., De la Fuente J., Sacchini S., … and Arbelo M. (2018). Retrospective study of foreign body-associated pathology in stranded cetaceans, Canary Islands (2000-2015). Environmental Pollution, 243, 519-527. https://doi.org/10.1016/j.envpol.2018.09.012
Rogan, E., Cañadas, A., Macleod, K., Begoña Santos, M., Mikkelsen, B., Uriarte, A., … and Hammond, P. S. (2017). Distribution, abundance and habitat use of deep diving cetaceans in the North-East Atlantic. Deep Sea Research Part II: Topical Studies in Oceanography, 141, 8-19. https://doi.org/10.1016/j.dsr2.2017.03.015
Rogan, E., Breen, P., Mackey, M., Cañadas, A., Scheidat, M., Geelhoed, S. C. V. and Jessopp, M. (2018). Aerial Surveys of Cetaceans and Seabirds in Irish waters: Occurrence, distribution and abundance in 2015-2017. https://secure.dccae.gov.ie/downloads/SDCU_DOWNLOAD/ObSERVE_Aerial_Report.pdf
Secchi, E. R. and Zarzur, S. (1999). Plastic debris ingested by a Blainville’s beaked whale, Mesoplodon densirostris, washed ashore in Brazil. Aquatic Mammals, 25(1), 21-24. https://www.researchgate.net/publication/263203190_Plastic_debris_ingested_by_a_Blainville%27s_beaked_whale_Mesoplodon_densirostris_washed_ashore_in_Brazil
Thompson, K. F., Patel, S., Baker, C. S., Constantine, R. and Millar, C. D. (2016). Bucking the trend: genetic analysis reveals high diversity, large population size and low differentiation in a deep ocean cetacean. Heredity, 116, 277-285. https://doi.org/10.1038/hdy.2015.99
Cuvier’s beaked whale
Aguilar de Soto, N., Gkikopoulou, K. C., Hooker, S., Isojunno, S., Johnson, M., Miller, P., … and Thomas, L. (2016). From physiology to policy: a review of physiological noise effects on marine fauna with implications for mitigation. Proceedings of Meetings of Acoustics, 27, 040008. https://doi.org/10.1121/2.0000299
Baird, R. W. (2018). Cuvier’s Beaked Whale Ziphius cavirostris. In B. Würsig, J. G. M. Thewissen and K. M. Kovacs (eds.), Encyclopedia of Marine Mammals (third edition), 234-237. London, UK: Elsevier. https://doi.org/10.1016/B978-0-12-804327-1.00100-X
Bachara, W. and Øien, N. (2017). Stranding records of Cuvier’s beaked whales (Ziphius cavirostris) in Norway. Unpublished report to Cetal Fauna. Report WB2017/2. 2 pp. https://www.researchgate.net/publication/329220486_Bachara_W_and_Oien_N_2017_Stranding_records_of_Cuvier%27s_beaked_whales_Ziphius_cavirostris_in_Norway_Unpublished_report_to_Cetal_Fauna_Report_WB20172_2_pp
Bernaldo de Quirós, Y., Fernandez, A., Baird, R. W., Brownell, R. L., Aguilar de Soto, N., Allen, D., … and Schorr, G. (2019). Advances in research on the impacts of anti-submarine sonar on beaked whales. Proceedings of the Royal Society B, 286, 20182533. https://doi.org/10.1098/rspb.2018.2533
Brownlow, A., Davison, N. and ten Doeschate, M. (2018). Annual Report 2018 1 January to 31 December 2018 for Marine Scotland, SRUC Wildlife Unit Drummondhill Inverness. Scotland: Scottish Government. https://www.strandings.org/smass/publications/reports/SMASS_Annual_Report_2018.pdf
Cañadas, A. (2012). Ziphius cavirostris (Mediterranean). The IUCN Red List of Threatened Species 2012: e.T23211A2785108. www.iucnredlist.org
Coomber, F., Moulin, A., Tepsich, P. and Rosso, M. (2016). Sexing free-ranging adult Cuvier’s beaked whales (Ziphius cavirostris) using natural marking thresholds and pigmentation patterns, Journal of Mammalogy, 97(3), 879-890. https://doi.org/10.1093/jmammal/gyw033
D’Amico, A., Gisner, R. C., Ketten, D. R., Hammock, J. A., Johnson, C., Tyack P. L. and Mead J. G. (2009). Beaked whale strandings and naval exercises. Aquatic Mammals, 55(4), 452–472. https://doi.org/10.1578/AM.35.4.2009.452
Dalebout, M. L., Robertson, K. M., Frantzis, A., Engelhaupt, D., Mignucci-Giannoni, A. A., Rosario-Delestre, R. J. and Baker, C. S. (2005). Worldwide structure of mtDNA diversity among Cuvier’s beaked whales (Ziphius cavirostris): Implications for threatened populations. Molecular Ecology, 14, 3353-3371. https://doi.org/10.1111/j.1365-294X.2005.02676.x
Dolman, S., Pinn, E., Reid, R., Barley, J., Deaville, R., Jepson, P. and Simmonds, M. (2010). A note on the unprecedented strandings of 56 deep-diving whales along the UK and Irish coast. Marine Biodiversity Records, 3. https://doi.org/10.1017/S175526720999114X
Frantzis, A. (1998). Does acoustic testing strand whales? Nature London, 5(392), 29. https://doi.org/10.1038/32068
Heyning, J. E. (1989). Cuvier’s beaked whale Ziphius cavirostris G. Cuvier, 1823. In Ridgway, S. H. and Harrison, R. J. (eds.), Handbook of Marine Mammals, volume 4: River dolphins and the larger toothed whales, 289-308. London, UK: Academic Press
Heyning, J. E. and Mead, J. G. (1996). Suction Feeding in Beaked Whales: Morphological and Observational Evidence. Natural History Museum of Los Angeles County, 464, 1-12.
Hoelzel A.R. (1998). Genetic structure of cetacean populations in sympatry, parapatry, and mixed assemblages: Implications for conservation policy. The American Genetic Association, 89, 451-458. https://doi.org/10.1093/jhered/89.5.451
Jepson, P. D., Arebelo, M., Deaville, R., Patterson, I. A. P., Castro, P., Baker, J. R., … and Fernandez, A. (2003). Gas-bubble lesions in stranded cetaceans. Nature, 425, 575-576. https://doi.org/10.1038/425575a
Ketten, D.R. (2014). Sonars and strandings: are beaked whales the aquatic acoustic canary? Acoustics Today Summer, 46-56. Available at: Acoustics today.
MacLeod, C. D., Santos, M. B. and Pierce, G. J. (2003) Review of data on diets of beaked whales: evidence of niche separation and geographic segregation. Journal of Marine Biology, 83, 651-665. https://doi.org/10.1017/S0025315403007616h
McSweeney, D. J., Baird R. W. and Mahaffy S. D. (2007). Site fidelity, associations, and movements of Cuvier’s (Ziphius cavirostris) and Blainville’s (Mesoplodon densirostris) beaked whales off the island of Hawaii. Marine Mammal Science, 23(3), 666-687. https://doi.org/10.1111/j.1748-7692.2007.00135.x
New L. F., Moretti D. J., Hooker S. K., Costa D. P and Simmons S. E. (2013). Using energetic models to investigate the survival and reproduction of beaked whales (family Ziphiidae). PLoS ONE, 8(7), e68725. https://doi.org/10.1371/journal.pone.0068725
Rogan, E., Cañadas, A., Macleod, K., Begoña Santos, M., Mikkelsen, B., Uriarte, A., … and Hammond, P. S. (2017). Distribution, abundance and habitat use of deep diving cetaceans in the North-East Atlantic. Deep Sea Research Part II: Topical Studies in Oceanography, 141, 8-19. https://doi.org/10.1016/j.dsr2.2017.03.015
Ross, G. J. B. (2006). Review of the conservation status of Australia’s smaller whales and dolphins Australian Government. Available at: Department of the Environment and Heritage
Schorr, G. S., Falcone, E. A., Moretti, D. J. and Andrews, R. D. (2014). First Long-Term Behavioral Records from Cuvier’s Beaked Whales (Ziphius cavirostris) Reveal Record-Breaking Dives. PLoS ONE, 9(3), e92633. https://doi.org/10.1371/journal.pone.0092633
Taylor, B. L., Baird, R., Barlow, J., Dawson, S. M., Ford, J., Mead, J. G., … and Pitman, R. L. (2008). Ziphius cavirostris. The IUCN Red List of Threatened Species 2008: e.T23211A9429826. https://doi.org/10.2305/IUCN.UK.2008.RLTS.T23211A9429826.en
Tyack, P. L., Johnson, M., Aguilar Soto, N., Sturlese, A. and Madsen, P. T. (2006). Extreme diving of beaked whales. The Journal of Experimental Biology, 209, 4238-4253. https://doi.org/10.1242/jeb.02505
Blainville’s beaked whale
Claridge, D.E. (2013).Population Ecology of Blainville’s beaked whales (Mesoplodon densirostris). Ph.D. thesis in Biology. University of St Andrews, Scotland. Available at: St.Andrews.
Dunn, B. P. (2015). Insights into Blainville’s Beaked Whale (Mesoplodon densirostris) Communication. Ph.D. thesis, University of St Andrews, Scotland. Available at: St. Andrews.
Ellis, R. and Mead, J. G. (2017). Beaked Whales: A complete guide to their biology and conservation. 194 pp. US: James Hopkins University Press
Hooker S.K., Aguilar de Soto N., Baird R.W., Carroll E.L., Claridge D., Feyrer L., … and Whitehead H. (2019). Future directions in research on beaked whales. Frontiers in Marine Science, 5(514). https://doi.org/10.3389/fmars.2018.00514
Jefferson, T., Webber, M. and Pitman, R. (2008). Marine Mammals of the Word: A Comprehensive Guide to Their Identification. London, England: Elsevier Publications. https://doi.org/10.1016/B978-0-12-804327-1.00172-2
Jepson, P. D., Arebelo, M., Deaville, R., Patterson, I. A. P., Castro, P., Baker, … and Fernandez, A. (2003). Gas-bubble lesions in stranded cetaceans. Nature, 425, 575-576. https://doi.org/10.1038/425575a
Pitman, R. (2018). Mesoplodon Beaked Whales. In B. Würsig, J. G. M. Thewissen and K. M. Kovacs (eds.), Encyclopedia of Marine Mammals (third edition), 595 – 601. London, UK: Elsevier. https://doi.org/10.1016/B978-0-12-804327-1.00172-2
Taylor, B. L., Baird, R., Barlow, J., Dawson, S. M., Ford, J., Mead, J.G., … and Pitman, R.L. (2008). Mesoplodon densirostris. The IUCN Red List of Threatened Species 2008: e.T13244A3426474. https://doi.org/10.2305/IUCN.UK.2008.RLTS.T13244A3426474.en
Sowerby’s beaked whale
Brownlow, A. Davison, N. and ten Doeschate, M. (2018). Annual Report 2018 1 January to 31 December 2018 for Marine Scotland, SRUC Wildlife Unit Drummondhill Inverness, Scotland: Scottish Government. Available at: SMASS
Carlström, J., Denkinger, J., Feddersen, P. and Øien, N. (1997). Record of a new northern range of Sowerby’s beaked whale (Mesoplodon bidens). Polar Biology, 17, 459-461. https://doi.org/10.1007/s003000050141
COSEWIC. (2006). COSEWIC assessment and update status report on the Sowerby’s beaked whale Mesoplodon bidens in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. 20 pp.
Cox, T. M., Ragen, T. J., Read, A. J., Vos, E., Baird, R. W., Balcomb, K., … and Benner, L. (2006). Understanding the impacts of anthropogenic sound on beaked whales. Journal of Cetacean Research and Management, 7(3), 177-187. https://archive.iwc.int/pages/search.php?search=%21collection15&k=
Culik, B. (2003). Odontocetes – the Toothed Whales – TS No. 24. Convention on the Conservation of Migratory Species of Wild Animals (Online issue). Bonn, Germany: CMS Secretariat. Available at: CMS
Ellis, R. and Mead, J.G. (2017). Beaked Whales: A Complete Guide to Their Biology and Conservation. Baltimore, US: Johns Hopkins University Press. https://doi.org/10.1353/book.52552
Hooker, S. K. and Baird, R. W. (1999). Observations of Sowerby’s Beaked Whales, Mesoplodon bidens, in the Gully, Nova Scotia. Canadian Field-Naturalist, 113(2), 273-277.
McGovern, B., Culloch, R., O’Connell, M. and Berrow, S. (2016). Temporal and spatial trends in stranding records of cetaceans on the Irish coast, 2002–2014. Journal of Marine Biology Association, UK. https://doi.org/10.1017/S0025315416001594
MacLeod, C. D. (2000). Review of the distribution of Mesoplodon species (order Cetacea, family Ziphiidae) in the North Atlantic. Mammal Review, 30, 1-8. https://doi.org/10.1046/j.1365-2907.2000.00057.x
MacLeod, C. D., Santos, M. B. and Pierce, G. J. (2003). Review of data on diets of beaked whales: evidence of niche separation and geographic segregation. Journal of the Marine Biological Association of the United Kingdom, 83(3), 651-665. https://doi.org/10.1017/S0025315403007616h
Mead, J. G. (1989). Beaked whales of the genus Mesoplodon. In S. H. Ridgway and R. Harrison (eds.), Handbook of marine mammals, Vol. 4: River dolphins and the larger toothed whales, 349-430. Academic Press. https://doi.org/10.1017/S0025315403007616h
Pereira, J. N., Neves, V., Prieto, R., Silva, M.A., Cascão, I., Oliveira, C., … and Clarke, D. (2011). Diet of mid-Atlantic Sowerby’s beaked whales Mesoplondon bidens. Deep Sea Research Part I: Oceanographic Research Papers, 58, 1084-1090. https://doi.org/10.1016/j.dsr.2011.08.004
Pitman, R. (2018). Mesoplodon Beaked Whales. In B. Würsig, J. G. M. Thewissen and K. M. Kovacs (eds.), Encyclopedia of Marine Mammals (third edition), 595 – 601. London, UK: Elsevier. https://doi.org/10.1016/B978-0-12-804327-1.00172-2
Reeves, R. R., Smith, B. D., Crespo, E. A. and Notarbartolo di Sciara, G. (2003). Dolphins, Whales and Porpoises: 2002-2010 Conservation Action Plan for the World’s Cetaceans. IUCN, Gland, Switzerland and Cambridge, UK. https://doi.org/10.2305/IUCN.CH.2003.SSC-AP.2.en
Rogan, E., Cañadas, A., Macleod, K., Begoña Santos, M., Mikkelsen, B., Uriarte, A., … and Hammond, P. S. (2017). Distribution, abundance and habitat use of deep diving cetaceans in the North-East Atlantic. Deep Sea Research Part II: Topical Studies in Oceanography, 141, 8-19. https://doi.org/10.1016/j.dsr2.2017.03.015
Taylor, B.L., Baird, R., Barlow, J., Dawson, S.M., Ford, J., Mead, J.G., Notarbartolo di Sciara, G., Wade, P. and Pitman, R.L. (2008). Mesoplodon bidens. The IUCN Red List of Threatened Species 2008: e.T13241A3424903. https://doi.org/10.2305/IUCN.UK.2008.RLTS.T13241A3424903.en
Tyack, P. L., Jonson, M., Aguilar de Soto, N., Sturlese, A. and Madsen, P.T. (2006). Extreme diving of beaked whales. Journal of Experimental Biology, 209, 4238-4253. https://doi.org/10.1242/jeb.02505
Waring, G. T., Hamazaki, T., Sheehan, D., Wood, G. and Baker, S. (2001). Characterization of beaked whale (Ziphiidae) and sperm whale (Physeter macrocephalus) summer habitat in shelf-edge and deeper waters off the northeast US. Marine Mammal Science, 17(4), 703-717. https://doi.org/10.1111/j.1748-7692.2001.tb01294.x
True’s beaked whale
Aguilar de Soto, N., Martin, V., Silva, M., Edler, R., Reyes, C., Carrillo, M., … and Carroll, E. (2017). True’s beaked whale (Mesoplodon mirus) in Macaronesia. PeerJ, 5. https://doi.org/10.7717/peerj.3059
Dalebout, M., Baker, C. S., Steel, D., Robertson, K. M., Chivers, S. J., Perrin, W. F., … and Schofield Jr, T.D. (2007). A divergent mtDNA lineage among Mesoplodon beaked whales: Molecular evidence for a new species in the tropical Pacific? Marine Mammal Science, 23(4), 954-966. https://doi.org/10.1111/j.1748-7692.2007.00143.x
Jefferson, T. A, Pitman, R. L. and Webber, M. A. (2015). True’s beaked whale. In T. A. Jefferson, R. L. Pitman and M. A. Webber (eds.), Marine mammals of the world (2nd edition), 135-138. Amsterdam: Elsevier.
MacLeod, C. D., Santos, M. B., Lopez, A. and Pierce, G. J. (2005). Relative prey size consumption in toothed whales: implications for prey selection and level of specialization. Marine Ecology Progress Series, 326, 295-307. https://doi.org/10.3354/meps326295
Macleod, C. D., Perrin, W. F., Pitman, R. L., Barlow, J., Balance, L., D’amico, … and Waring, G. T. (2006). Known and inferred distributions of beaked whale species (Ziphiidae: Cetacea). Journal of Cetacean Research and Management, 7(3), 271-286. https://archive.iwc.int/pages/search.php?search=%21collection15&k=
Pitman, R. (2018). Mesoplodon Beaked Whales. In B. Würsig, J. G. M. Thewissen and K. M. Kovacs (eds.), Encyclopedia of Marine Mammals (third edition), 595 – 601. London, UK: Elsevier. https://doi.org/10.1016/B978-0-12-804327-1.00172-2
Robbins, J. R, Park, T. and Coombs, E. J. (2019). Supernumerary teeth observed in a live True’s beaked whale in the Bay of Biscay. PeerJ, 7. https://doi.org/10.7717/peerj.7809
Ross, G. J. B. (1969). Evidence for a southern breeding population of True’s beaked whale. Nature, 22(58). https://doi.org/10.1038/222585a0
Silva, M. A., Prieto, R., Cascão, I., Seabra, M. I., Machete, M., Baumgartner, M. F. and Santos, R, S. (2014). Spatial and temporal distribution of cetaceans in the mid-Atlantic waters around the Azores. Marine Biology Research, 10, 123-137. https://doi.org/10.1080/17451000.2013.793814
Taylor, B. L., Baird, R., Barlow, J., Dawson, S. M., Ford, J., Mead, J. G., … and Pitman, R. L. (2008). Mesoplodon mirus. The IUCN Red List of Threatened Species 2008: e.T13250A3430702. https://doi.org/10.2305/IUCN.UK.2008.RLTS.T13250A3430702.en
Vonk, R. and Martin, V. (1988). First list of Odontoceti from the Canary Islands, 1980–87. In P. G. H. Evans (ed.), 2nd Annual Conference of the European Cetacean Society.
Weir, C. R., Stokes, J., Martin, C. and Cermeño, P. (2004). Three sightings of Mesoplodon species in the Bay of Biscay: first confirmed True’s beaked whales (M. mirus) for the north-east Atlantic? Journal of the Marine Biological Association of the UK, 84, 1095-1099. https://doi.org/10.1017/S0025315404010525h



