Sperm Whale
Latest update: July 2021
Sperm whales are the largest of the toothed whales, only surpassed in size by the blue whale and other large rorquals. They can be found in all the world’s oceans, although they prefer deeper water around the continental shelf. Their diet consists primarily of deep-sea cephalopods including the giant squid. Sperm whales are among the animal kingdom’s deepest divers, consistently reaching depths of 1000m or more to hunt. They have a long maturation process, and females and juveniles group together in small family units. Males tend to be solitary and range into higher latitudes than females. Once the target of intense hunting for their highly-prized spermaceti oil, they are now a common sight for whale-watchers and can be seen from popular spots such as Vesterålen in Norway.
ABUNDANCE
Around 30,000 sperm whales have been estimated in the North Atlantic, with approximately 300,000 worldwide.
DISTRIBUTION
Sperm whales have a cosmopolitan distribution up to the ice-edge at both poles (i.e. their range extends across most of the world in appropriate habitats). They are most abundant in deep waters with high productivity.
RELATION TO HUMANS
Sperm whales were highly sought after during the period of industrialised whaling (18th century and onwards) because the liquid wax (sperm oil) they produce was a particularly useful illuminant and lubricant at the time.
They are now a popular species for whale-watching tours, such as those off Vesterålen in Norway.
CONSERVATION AND MANAGEMENT
Population structure for sperm whales has been difficult to determine, yet in the North Atlantic their numbers appear to be increasing. Management is currently conducted by NAMMCO and the IWC. The species is protected in all NAMMCO member countries.
In the most recent assessment (2023) the species is listed as ‘Vulnerable’ on the regional European IUCN Red List and Greenlandic national red list, while Icelandic national red list list species as ‘Data Deficient’.

Sperm whale as it appears on a Faroese postage stamp from 2001
Scientific name: Physeter macrocephalus
Faroese: Avgustur
Greenlandic: Kigutilissuaq
Icelandic: Búrhvalur
Norwegian: Spermhval, spermasetthval
Danish: Kaskelothval
English: Sperm whale, cachalot
LIFESPAN
It is thought that sperm whales live to around 70 years or more.
SIZE
Males are larger than females. Mature females are on average 11m long, while males can reach 16m or more.
MIGRATION AND MOVEMENTS
The movement and migration of sperm whales is not well-understood in detail. However there is an apparent seasonal migration polewards in the summer by males, while many females tend to stay at lower latitudes with no apparent movement pattern.
FEEDING
Sperm whales primarily feed on deep-sea cephalopods (i.e. squid, cuttlefish, octopuses). Males are more likely than females to also prey on fish species such as demersal elasmobranchs and gadoids.
Foraging occurs on long dives (an hour or more) to maximum depths of 1-3km.
General Characteristics
Sperm whales are the largest of the odontocete (‘toothed’) whales, a group that also includes dolphins, porpoises, and beaked whales. They are the only extant member of the genus Phsyeter, with two other species of the genus Kogia (the pygmy and dwarf sperm whales), thought to be their closest relatives (Wilson & Reeder 2005, Whitehead 2018).
At Sea
Sperm whales are physically distinct from other large cetaceans and are largely unmistakeable to whale-watchers due to their unique shape (Shirihai & Jarrett 2006). The species name, macrocephalus, is derived from a Greek word literally meaning ‘big-headed’ and their characteristically large square head can represent a third of their total length. Their blows are also directed out of a single blowhole on the left side of their snout.
Sperm whales are accomplished divers and may be underwater for an hour or more. Between dives they can appear slow and docile. When seen at the ocean’s surface, their long body is often motionless or ‘logging’. Before a deep dive, sperm whales arch their backs and raise their tails out of the water so that their flukes are visible.
Physical Traits
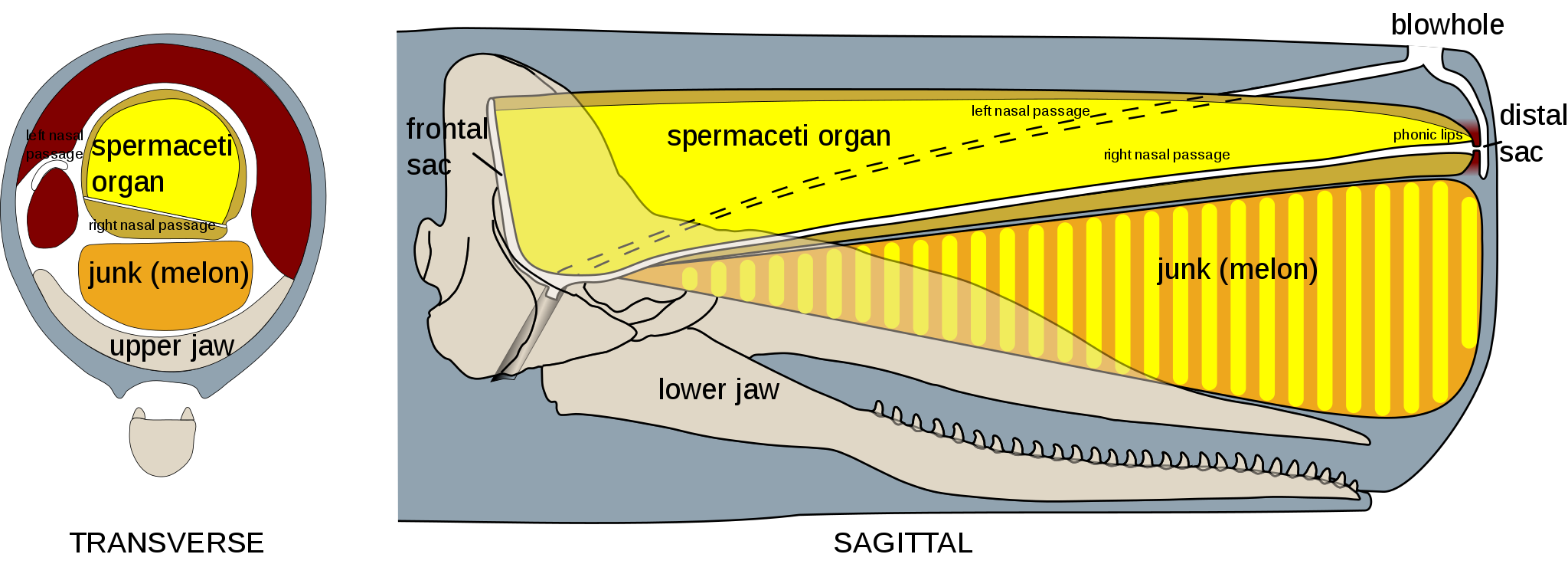
The region above the sperm whale’s lower jaw contains a large nasal complex and includes the spermaceti organ – containing the once highly prized oil from where the species derives its name (Whitehead 2018).
The species has a narrow, underslung lower jaw with 18-26 teeth on each side. These fit into sockets in the upper jaw (Jefferson et al. 2008). They possess small, paddle shaped flippers and a low dorsal fin. The blowhole is asymmetrical and positioned on the left anterior part of the head. The coloration is a uniform dark grey, yet lighter patches may appear under the belly and around the jaw and become more visible with age. The skin towards the tail can have a wrinkled appearance, but is smooth over the head and on the tail itself (Whitehead 2018).

Sperm Whale Diving ©Malene Thyssen
Sperm whales are sexually dimorphic, with males being larger than females. On average mature males reach 16 metres long and weigh around 45 tons. Larger specimens have been found reaching almost 20 meters and weighing 57 tons (Jefferson et al. 2008). Mature females are on average around 11 meters long and weigh 15 tons.
The species has a cosmopolitan distribution and is found all the way up to the ice-edge at the poles. Both males and females can be found in the tropics and below around 40° latitude. However, only the larger males tend to be found at high latitudes. Both population structure and migrations are poorly understood, yet there appears to be a general poleward movement in the summer months from mid latitudes

Life History and Ecology
Sperm whales have a long lifespan, late maturity, low reproductive rate and a high juvenile survival rate. Total lifespan can be up to 70 years, with females reaching sexual maturity at around nine years of age, while males mature between the ages of 10-20 years (Whitehead 2018). While males have a longer duration of puberty, they may not even be sexually active until their late twenties. Adult females will usually give birth to single calves at least five years apart. The gestation period lasts 14-16 months, and calves suckle for between 19-42 months (Shirihai and Jarrett 2006).
Breeding occurs in the tropics during the summer months, with males travelling from higher latitudes to find and join the reproductive females. Females give birth in the summer or autumn of the following year, with calves remaining close to their mothers in a social group while suckling. Calves may also suckle from mature females other than their mothers (Shirihai and Jarrett 2006).
Echolocation and Communication
Sitting above the jaw of the sperm whale lies a complex system that enable echolocation. Sound is generated in the form of clicks from a structure known as the museau de singe, or ‘monkey lips’, which are positioned anteriorly (Zimmer et al. 2005). Soundwaves then pass backwards through the spermaceti organ, a large tissue structure containing up to 1,900 litres of waxy esters and triglycerides that form ‘spermaceti oil’ (WDCS 2010). A frontal air sac between the spermaceti organ and the cranium then reflects the soundwaves forward through a structure below the spermaceti called the ‘junk’.
The junk, which is comparable to the melon of other odontocetes, comprises a series of cartilaginous compartments containing more spermaceti oil. These compartments act like lenses to focus the soundwaves forward and out. The returning echoes are received by the lower jaw, which transmits the vibrations along a fat-filled channel to the inner ear (Norris and Harvey 1972).
The sounds emitted during echolocation can be classified into distinct patterns, each with a different purpose. Almost all are in the form of clicks used in foraging and social behaviour, yet occasionally ‘squeals’ and ‘trumpeting’ sounds have also been recorded (Whitehead 2018). Clicks in the form of high frequency creaks are thought to be used in tracking and homing in on prey; with longer intervals between clicks when the sperm whale is searching, or shorter intervals just prior to prey capture. Lower frequency ‘codas’ are thought to be used in social interactions and have distinctive durations and intervals that may be specific to a social unit. For example, a low frequency ‘slow’ click is used solely by males (Madsen et al. 2002, Whitehead 2003).
Sperm Whale Vocalisations:
Here you can find examples of different vocalisations made by sperm whales. The different sounds each have a purpose – either for communication or for feeding:
Ordinary ‘Clicks’ used to search for prey:
Source: published under the Creative Commons CC0 1.0 Universal Public Domain Dedication
Creaks made when homing in on prey:
Source: NOAA, licensed as public domain
Codas – click sequences made by females to communicate:
Source: Jim Nollman, licensed under the Creative Commons Attribution-Share Alike 3.0 Unported license
Slow clicks made by males to communicate:
Source: NOAA, licensed as public domain
Feeding Behaviour
Feeding occurs during long dives that typically range between 300-800 metres deep, although they may be up to 2 km and last over an hour (Whitehead 2003). Various anatomical and physiological features enable such diving, such as increased myoglobin concentrations in the muscles, bradycardia (the slowing of the heart rate), and a collapsible ribcage that allows lung compression at depth without causing injury. The limits of the deep-diving behaviour of sperm whales was first partially understood when a whale was found entangled in a telephone cable at 1,300 metres depth.
Diet
The sperm whale diet consists of a wide variety of species, with the majority taken during long foraging dives to the seafloor (Clapham 1997). The diet primarily consists of cephalopods (i.e. squid, cuttlefish, octopuses), of which medium sized species are thought to comprise the largest proportion (Whitehead 2003). Faecal sample analysis conducted in the Galápagos Islands found that 62% of cephalopod beaks retrieved belonged to the genus Histioteuthis ‘cock-eyed’ squids. Other important genera found were the Ancistrocheirus (16%), and the Octopoteuthis (7%) (Smith and Whitehead 2000).
Both males and females are known to prey on cephalopods, especially where adults of both sexes occur at mid-latitudes. They are known to predate upon giant squid belonging to the genus Architeuthis, and many bear the scars of their encounters. Mature males venturing closer to the Antarctic coast have been discovered with beaks belonging to the colossal squid, (Mesonychoteuthis hamiltoni) in their stomachs (Clarke 1980, Whitehead 2018). This species inhabits the deep waters of the Southern Ocean, and may grow up to 14 metres. The hooked tentacles and serrated suckers off large squid species are thought to be responsible for much of the scarring seen on the rostrums of large sperm whales.
Males are more likely than females to also feed on demersal fish and chondrichthyan species in higher latitudes. A stomach contents analysis of 221 adult male sperm whales off the coast of Iceland revealed that fish remains were found in 87% of samples, while the proportion containing cephalopods was only 68%. The lump sucker, Cyclopterus lumpus, was found to be a preferred prey species (Whitehead 2018, Martin and Clarke 2019).
Did you know?
New species of deep-sea squid are often first described from beaks found inside the stomachs of dead sperm whales!

The sperm whale (Physeter macrocephalus) or cachalot is the largest of the toothed whales and the largest toothed predator. It is the only living member of genus Physeter and one of three extant species in the sperm whale family. © Dmitry Kokh, iStock
Social Organisation
Mothers, calves and juveniles will form social groups, with young males also forming so-called ‘bachelor’ groups. Large adult bulls are generally solitary yet will join a group or visit a harem during the breeding season.
Female sperm whales are known to have strong social bonds. Pods of up to 10 individuals form a family unit, which often consists of related adult females and their young (Whitehead 2003). Multiple family units may come together to form clans, often united by shared behavioural habits, as well as unique codas (see ‘Behaviour’). Clans may overlap in range with each other, although this is more common in the Pacific than the Atlantic (Gero et al. 2016).
Communal care for other females’ offspring exists within groups, with foraging behaviour often staggered so that young animals may be guarded near the surface. Males leave family units between the ages of 4 and 21, sometimes forming bachelor pods with other males, but gradually becoming more solitary with age (Whitehead 2018). Males will join female units during breeding periods, yet will not associate with each group for more than a few hours before moving on.
Predators
It had long been thought that the only predators capable of harming sperm whales were humans. Indeed, this species was highly prized by whalers throughout the period of industrialised whaling due to the large quantities of liquid wax that could be obtained from their spermaceti organ.
However, observations over recent decades have challenged this idea. Killer whales (Orcinus orca) are now known to harass and even kill adult sperm whales, and their role as important predators of this species may have been overlooked (Pitman et al. 2001, Whitehead 2018). Sperm whales may also respond defensively to the presence of other large oceanic dolphins such as pilot whales (Globicephala spp.) and false killer whales (Pseudorca crassidens).

Killer Whales ©Fernando Ugarte
Defensive response
Sperm whales are observed to adopt a ‘rosette’ formation in response to threats from other marine mammals. The rosette is formed by a group of whales facing inward in a circular pattern with their tails oriented outward. This behaviour is thought to be an effective way for the whales to defend vulnerable or injured individuals, and provide the threat of a heavy blow from the outward facing tail flukes.
However, in one documented attack on a group of female or juvenile sperm whales by a pod of at least 17 killer whales, the rosette formation was not successful (Pitman et al. 2001). One large whale was killed by the predators, while many others in the pod suffered serious and fatal injuries from the attack. The killer whales, most of them adult females and calves, made repeated charge and withdraw cycles, continually weakening all individuals until one was able to be killed. In apparent altruistic behaviour, when one sperm whale was pulled away from the rosette, other whales broke formation to help it back to the group. This made them vulnerable to attack and eventually resulted in all members of the pod suffering likely fatal injuries (Pitman et al. 2001).
Health – Diseases and Parasites
Autopsied sperm whales have been seen to contain a wide variety of pathogens and parasites. Samples and observations made from stranded animals, as well as from historic hunting records, have shown various infections and diseases, some of which may have been the ultimate or proximate cause of death (Berzin 1971, Lambertsen 1997).
Ectoparasites seen to infest sperm whales include diatoms, vertebrate parasites such as lampreys and shark suckers, as well as whale lice, anchor worms and stalked barnacles (Berzin 1971). Many of these are found on the epidermis and inside anal and genital slits, while barnacles tend to be associated with the animal’s teeth (Lambertsen 1997). Many endoparasites are also found, particularly in the digestive system. These include many trematode species (flukes), cestodes (tapeworms), and acanthocephalans (thorny-headed worms). Perhaps the most abundant endoparasites are nematodes (roundworms), particularly those belonging to the genus Anisakis.
Evidence of heart disease and myocardial infarctions has also been found in some individuals. This, along with ulcerations caused by the parasitic worm Anisakis, represent the most prominent natural causes of death in sperm whales. Viral diseases are also present, including Papillomavirus, which just like in humans, can lead to the development of tumours (Lambertsen 1997).
Distribution and Habitat
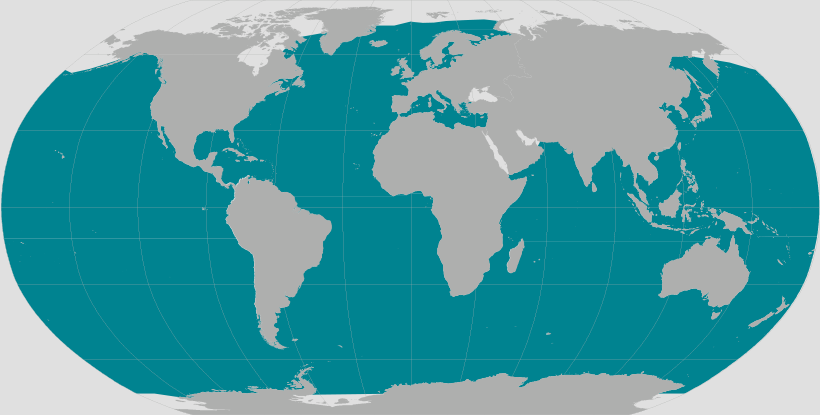
Sperm whales can be found in almost all the world’s oceans, although they typically prefer waters that are greater than 1000 metres deep. That said, their range extends from the tropics to the poles, often right up to the ice-edge (60°N – 70°S). Certain oceanographic features attract higher concentrations of individuals, with population density generally increasing near to the continental shelf and around seamounts.
Throughout the period of intensive commercial whaling, it was well known that there were highly productive ‘grounds’ where sperm whales were especially abundant (Whitehead 2003, 2018). These were often predicted by noticing sudden changes in sea-surface temperature and the presence of ocean fronts became a signal that whales were near-by (Jaquet 1996).
The exact association with fronts has been subject to debate since direct correlations with oceanographic features have not always been consistent. Some argue that upwelling zones contribute to high population densities due to increased environmental productivity (Best 1969, Clarke et al. 1978, Jaquet 1996). Alternatively, it has been suggested that sperm whales congregate in downwelling zones. Berzin (1971) argued that because the downwelling process brings zooplankton and oxygen to deeper water, it promotes biomass increase of sperm whale prey, i.e. deep-water cephalopods. Despite these contradictory positions, it has generally been concluded that sperm whales tend to associate with areas of high planktonic biomass, yet the spatial scales at which these associations are detected are large – in the order of hundreds of nautical miles (Jaquet 1996).
Distribution also varies between sexes. Older males tend to venture to higher latitudes while females and juveniles more commonly remain in the tropics.
In the North Atlantic
Sperm whales are widely distributed throughout the North Atlantic, yet are found at higher densities in areas such as the Bay of Biscay, to the west of Iceland, and towards northern Norway (Rogan et al. 2017). Further south, they can also be found in large numbers around the Azores.
Most sperm whales that are seen at higher latitudes are solitary males, with females generally remaining further south. Popular whale-watching locations for sperm whales include Vesterålen in Norway, and the waters surrounding the archipelago of the Azores.
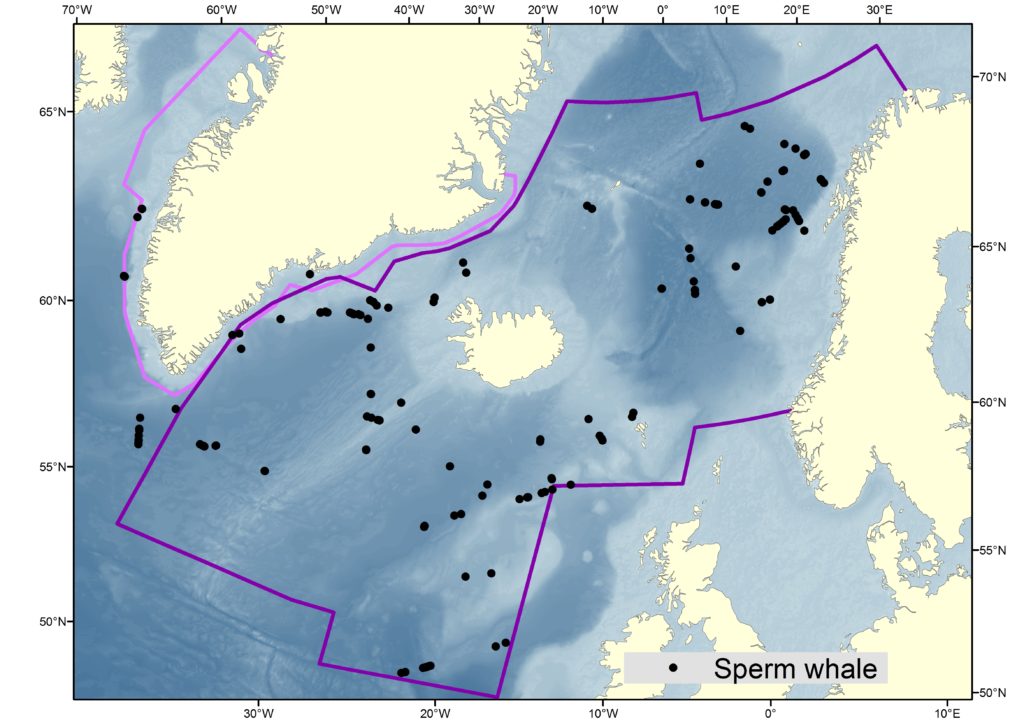
Migration and Movements
The exact migration and movement patterns of sperm whales are not particularly well understood. Adult males are generally found at higher latitudes than females, which implies the occurrence of poleward migrations following breeding periods spent at lower latitudes.
Sperm whales are long-lived species with low reproductive rates i.e. they possess typically K-selected life-histories. As such, they are unable to capitalise on increased food abundance by maximising reproductive output unless over very large spatial and temporal scales. Tracking environmental variation is therefore critical to their survival if they are also to a maintain healthy biomass and population sizes. Since environmental variation in productivity can be considerable, migration to new grounds is one way in which sperm whales can survive this uncertainty. Whitehead (1996) found that in times of local food shortages, animals travelled faster, further, and on more direct pathways than when food was plentiful. Tracked individuals have been found to travel over 1000km in search of food, and may travel half of that distance in only 5-6 days (Whitehead 1996).
Given the social groups formed by females and juveniles, memory has been theorised as playing a part in judging the probability of success when moving to a new area to feed. The importance of memory and ‘sharing’ information about the environment has been suggested to be an important part of elephant behaviour, an animal that shares many of the K-selected traits with sperm whales (Eisenberg et al. 1971, Whitehead 1996, Baskaran et al. 2018). Information regarding migration in sperm whales may therefore also support the existence of long-term bonds between members of a pod.
Error in navigation causing trouble
However, migratory behaviour may also lead sperm whales into danger. The North Sea, a relatively shallow marginal sea in the North Atlantic with an average depth of only 90 metres, is known as a hotspot for frequent sperm whale strandings along the British, Dutch and Danish Coastline. It is thought that males returning to the Azores from the Northeast Atlantic accidentally track eastwards past the British Isles, rather than West through deeper water. The shallow North Sea does not give the whales access to the deep-water species that they prey upon. This leads to a loss of body condition and eventual starvation as they struggle to feed during their journey south (Lambertsen 1997, Unger et al. 2016).
North Atlantic Stocks
While the exact structure of the population is not fully understood, it’s thought that most animals observed by NAMMCO member countries in the North Atlantic are male. This is due to the sex segregation of the species, with males distributing themselves at higher latitudes than females and juveniles (Teloni et al. 2007, Rogan et al. 2017, Whitehead 2018).
Older males are generally solitary in the North Atlantic, although so-called “bachelor herds” consisting of younger males do occur. Since males migrate southwards to breed with females in warmer waters, the degree of reproductive isolation in the North Atlantic is uncertain.
There is still a broadly low genetic diversity for this species, which is potentially the result of an historic genetic bottleneck.
For the purposes of stock identification, it may be appropriate to consider the social structure and use clans as indicators of population divisions (Whitehead 2018). Clans may have unique behavioural traits, such as specific codas, that can be used to assign individuals to specific groups and perhaps genetic lineages (Rendell and Whitehead 2005). The fact that clans in the Atlantic are less likely to overlap in their distribution than in the Pacific would be an advantage to stock identification, however, this may only be possible for females (Gero et al. 2016).
Estimating Sperm Whale Abundance
Sperm whales are a challenging species to estimate the abundance of. Since they dive for long periods of time, it is likely that many animals may be missed during sighting efforts along the transect lines, which would lead to a negatively biased estimate (Gunnlaugsson et al. 2009, Rogan et al. 2017, Hammond et al. 2017, Pike et al. 2019). Trials of using hydrophones (microphones detecting soundwaves underwater) to develop abundance estimates have been conducted and may be an effective method of obtaining more accurate data (Rogan et al. 2017). Although hydrophones are able to detect animals underwater to make up for those missed at the surface, this method is not yet widely implemented for estimating abundance.
Abundance in the Northeast Atlantic
From a series of surveys (from 1996-2007) it is estimated that there may be as many as 30,000 sperm whales in the Northeast Atlantic. As there is limited overlap in the key survey areas, and there is likely a negative bias due to sperm whale diving behaviour, this figure may be quite conservative (Pike et al. 2019). This is compared to an estimated 300,000 animals worldwide (Shirihai and Jarrett 2006).
The surveys conducted may not have targeted sperm whales specifically, yet recorded sightings have helped generate abundance estimates for the species.
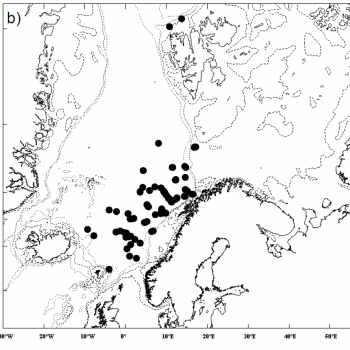
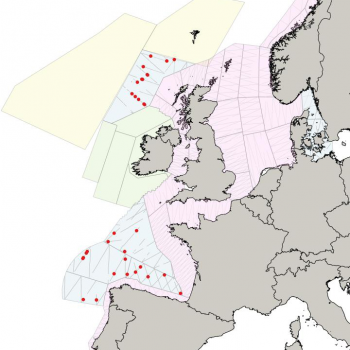
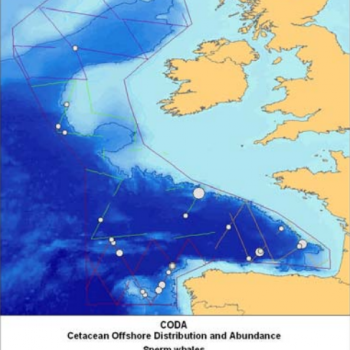
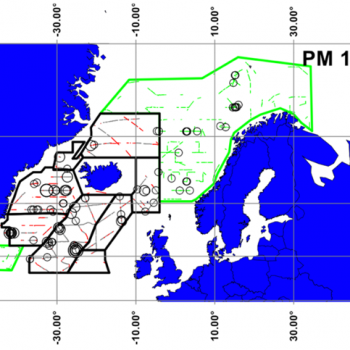
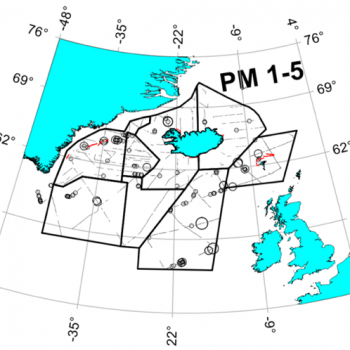
Estimates from various sources are displayed in the the table below, and the maps also help to illustrate the distribution of sperm whale sightings.
Abundance Estimates for Sperm Whales in the Northeast Atlantic
| Survey | Year | Area | Estimate | 95% Confidence Interval | Source |
|---|---|---|---|---|---|
| NASS | 2001 | Iceland/Faroes | 11,185 | (CV = 0.34) | Gunnlaugsson et al. 2009 |
| NASS | 2007 | Iceland/Faroes | 12,220 | 5,807 – 25,717 | NAMMCO 2018 |
| NASS | 2015 | Iceland/Faroes | 23,166 | 7,699 – 69,709 | NAMMCO 2018, Pike et al. 2019 |
| Norway | 1996-2001 | Norway and Adjacent Waters | 6,375 | 4,163 – 8,762 | Øien 2009 |
| Norwegian Mosaic | 2002-2007 | Norway and Adjacent Waters | 8,134 | 5,695 – 11,617 | NAMMCO 2019 |
| Norwegian Mosaic | 2008-2013 | Norway and Adjacent Waters | 3,962 | 2,218-7,079 | NAMMCO 2019 |
| Norwegian Mosaic | 2014-2018 | Norway and Adjacent Waters | 5,522 | 3,325 – 9,170 | NAMMCO 2019 |
| SCANS-III | 2016 | European Coastal Waters | 13,518 | 6,181 – 29,563 | SCANS-III 2017 |
| CODA | 2007 | European Offshore Waters | 2,077 | 1,404 – 3,073 | CODA 2009 |
The NASS-2015 Icelandic and Faroese ship survey generated an estimate of around 23,000 animals, with the greatest densities around the Faroe Islands and to their southwest (Pike et al. 2018). Øien (2009) estimated around 6,000 animals from the most recent surveys of Norwegian waters, with the largest number of sightings being over the continental slope in Northern Norway. Further south, the SCANS-III Survey conducted in the summer of 2016 provided an estimate of nearly 15,000 sperm whales, and like the CODA survey from 2007, found a large concentration around the Bay of Biscay.
Maps
Identification and Structure
Stock identification is an important aspect of management and conservation. A stock is generally defined as a reproductively isolated sub-unit of a population; there is limited gene-flow between animals belonging to different stocks. If this isolation is maintained, they may become genetically distinct and share morphological, behavioural, and life-history traits only with those belonging to the same stock. Successful management of a species relies on accurate stock identification that best represents the dynamics of the population being monitored (Begg and Waldman 1999).
The population structure of sperm whales, like their habitat associations and migration patterns, is uncertain. Studies of genetic diversity have found little variation in nuclear microsatellites between geographic locations. However, mitochondrial DNA does show significant variation between ocean basins. This is a typical indication of gender-biased dispersal; wider-ranging males versus more geographically constrained females (Schrey and Heist 2003, Alexander et al. 2016, Whitehead 2018). The Mediterranean may be a genetically distinct population due to geographic segregation (Notarbartolo-Di-Sciara 2014).
Management in the North Atlantic
Sperm whales have been protected globally since the introduction of the International Whaling Commission’s (IWC) moratorium in 1986. This moratorium imposed a temporary ban on all commercial whaling, while allowing for some aboriginal subsistence whaling and whaling for scientific purposes. Japan has taken up to 10 sperm whales per year since 2000 as part of their scientific research program, while two villages in Indonesia continue to engage in subsistence whaling and take a similar number for consumption (Taylor et al. 2008, Whitehead 2018).
Sperm whales currently fall under the management of both NAMMCO and the IWC. In all NAMMCO member countries sperm whales have a fully protected status.
Sperm whales have a very low population growth rate, potentially as low as 1% per year (Whitehead 2003, Taylor et al. 2008, Chiquet et al. 2013). This makes them susceptible to threats because they can take a long time to recover from any removals. Knowledge gaps concerning population structuring in the North Atlantic (and globally) also create challenges for developing effective management strategies.
International Status
IUCN (International Union for Conservation of Nature): Vulnerable
CITES (Convention on International Trade in Endangered Species of Wild Fauna and Flora): Appendix I (Commercial trade in this species is illegal)
CMS (Convention on the Conservation of Migratory Species of Wild Animals): Appendix I and Appendix II (threatened species also requiring international cooperation in conservation efforts)
Berne Convention (Convention on the Conservation of European Wildlife and Natural Habitats): Appendix II (Strictly protected Fauna – Mediterranean only)

Early Utilisation
Until around 300 years ago, sperm whales had not been widely exploited. Since they tend to prefer deep water, prior to this time it was rare to encounter sperm whales compared with other more accessible species. However, beginning in the early 18th century, the potential of whale oil was beginning to be realised. Growth during the industrial revolution relied heavily on processed whale oil (Whitehead et al. 1997, Whitehead 2018).

Capturing a Sperm Whale, William Page (1811-1885)
Whale oil was usually obtained by flensing (cutting away) the blubber, and then rendering it onboard the ship before storing it in barrels for transport. Often the meat was discarded.
Sperm whales were a particularly important target for whalers due to the spermaceti organ from which the animal gets its name. The enlarged head of a bull sperm whale could yield nearly 2000 litres of sperm oil, which was highly prized due to its superior quality when compared with regular whale oil obtained from the blubber.
Spermaceti oil required no additional processing and could be used to lubricate finer machinery. The waxy solids also found in the spermaceti organ were made into high-value candles that burned cleaner and longer than the more common tallow. Another product of the sperm whale fishery was Ambergris. This substance produced in the whale’s digestive system was used until recently as a fixative in perfume production. The superior nature of sperm whale products lead to sperm-whaling becoming a major global industry, initially centred around New England, but quickly spreading worldwide. By the mid 19th century, approximately 5000 whales were being killed annually.
Methods
Whaling took place from small whaleboats rowed from a main ship. Hunting often took place in specific ‘grounds’ known for higher densities of whales (See Distribution – Habitat). Once a whale was sighted, the whale-boats were lowered from the main ship and approached the whale. A harpoon with a long line attached was cast at the whale, at which point it would drag the boats in what was termed a ‘Nantucket sleighride’, named after the centre of the American whaling industry. This was particularly dangerous for the whalers as sperm whales were known drag boats at up to 23mph (37 km/h), and over longer distances than other species. Once the whale was exhausted, and if the crew had survived the chase, the whale would be lanced and towed back to the main ship for processing (Whitehead 2003).

Moby Dick Title Page
Occasionally, whales were known to fight back aggressively, sometimes attacking even the larger ship during the hunt. A famous example from 1820 was the fate of the Essex, a Nantucket whaler operating in the South Pacific. A large bull sperm whale rammed the hull of the Essex while hunting in the grounds off the coast of South America, causing her to sink. Only 8 of the 20 crew survived the following months adrift in lifeboats after abandoning ship.
The story of the Essex eventually went on to inspire Herman Melville’s 1851 novel Moby Dick, which has since become a literary classic.
20th Century
With the advent of petroleum processing and utilisation, whale oil became less important as a valued commodity for industrial development. However, sperm oil was still highly sought after due to its specific qualities. It was still very useful in devices such as transmission systems in cars, as well as for use in cosmetics and soaps. Furthermore, so-called ‘modern whaling’ began using all parts of the whale, not simply taking the oil but the meat as well (Whitehead 2018).
Modern whaling was also much more efficient at hunting whales. The introduction of steam and petroleum fueled factory ships, and the use of explosive harpoons fired from cannons, enable the species to be exploited at a much higher rate. Norwegian whalers operating off the coast of Peru in the early 1940s were alone able to capture over 3,000 sperm whales.

20th Century Whaling Cannon circa 1920
After World War II, hunts continued to capture up to 30,000 sperm whales per year globally. Rationing during the war lead to whale meat being marketed as a cheap source of animal protein and populations of many whale species, particularly in the Southern Ocean, began to dwindle. It is thought that pre-whaling populations of sperm whales numbered 1,100,000 animals worldwide (Taylor et al. 2008). Since the moratorium in the 1980s, and the establishment of the protected status (with a few exceptions), the species now numbers around 300,000 animals (Taylor et al. 2008).
Recovery
Certain aspects of the whales’ biology have hampered its recovery from industrial whaling. Like many large mammal species, it has a slow reproductive rate and low fecundity. Calves take a long time to mature and adults do not reach sexual maturity until they are a decade old or more. Even after being given protected status, sperm whale populations may only be increasing at a rate of 1% per year.
Even after the IWC’s moratorium on whaling in 1986, the lingering effect of over-hunting may still be seen in sperm whale populations. Whitehead et al. (1997) showed that animals in the Galapagos were still declining at around 20% per year between 1985 and 1995. Sperm whaling in this region stopped earlier than the moratorium, with the last whale caught in 1981, demonstrating the vulnerability of the species and that full recovery may not be possible everywhere (Chiquet et al. 2013). Long-term disruption to social structures caused by decades of whaling pressure may still have lingering effects upon populations and be inhibiting recovery (Whitehead et al. 1997).
21st Century
Currently, sperm whales are only caught in small numbers by a very limited number of countries and they are not taken for commercial purposes.
Since 2000, Japan has taken around 10 sperm whales per year as part of scientific research program.

Whale Hunt in Indonesia © Palani Mohan
Aboriginal subsistence hunting for consumption still occurs in Indonesia, from the villages of Lamalera and Lamakera. Fishermen here use small boats and hand-held harpoons to catch whales in the deep water around their islands. The catch is small (no more than 10 each year), and the whole animal is shared and used among the people of the village.
Threat Exposure
Although now protected from hunting (with exceptions for small subsistence and scientific research hunts), sperm whales still encounter anthropogenic threats that impact their survival. As they inhabit deep, offshore waters, they are perhaps less affected by these threats than more coastal species. However, they do encounter dangers such as by-catch (Notarbartolo-Di-Sciara 2014), ship-strikes (Laist et al. 2001) and pollution (Nielsen et al. 2000), and are further affected on a broader scale by climate change (MacLeod 2009).
By-catch, Entanglements and Ship Strikes
Sperm whales are can be become entangled in fishing gear and many die as by-catch. For the Mediterranean population – which inhabits an area of high fishing intensity – entanglement in swordfish and tuna driftnets has been considered their greatest threat. Between 1971 and 2004, Italy, France and Spain recorded 229 sperm whales for which entanglement was thought to be the cause of death (Notarbartolo-Di-Sciara 2014). Driftnets were outlawed in the Mediterranean in 2002, however, illegal fishing activity means that the threat still persists.
Across the globe in longline fisheries, odontocete depredation may account for over US$8,000 worth of losses daily (Hamer et al. 2012). Sperm whales and orca are thought to be the main culprits at higher latitudes, and increased interaction with these fisheries increases the chance of bycatch (Hamer et al. 2012). Deterrents have been trialed, both physical and acoustic, yet return mixed results (Barlow & Cameron 2003, Hamer et al. 2012).
As they are a comparatively slow-swimming species, and after deep dives they will often lie motionless in a behaviour known as ‘logging’, there are many accounts of ships hitting ‘sleeping’ whales (Laist et al. 2001).
Noise/Disturbance
Odontocetes in general are thought to be far more susceptible to the effects of noise pollution than baleen whales. This is due to sound interfering with their own noise production and echolocation, which they use for feeding and maintaining social bonds. Harris et al. (2015), reported behavioural disruption for sperm whales, pilot whales and killer whales as a result of naval sonar activity. Other studies have, however, reported little or no response (Madsen et al. 2002) and long term detrimental effects of noise have yet to be assessed.
Contaminants and Pollution

Beached Sperm Whale on the North Sea Coast
Sperm whales appear to have greater levels of chemical and heavy metal contaminants in their blubber than baleen whales. This is likely due to biomagnification as they feed at higher trophic levels than filter-feeding rorquals. However, they do have lower levels than many other odontocetes. This could be due to their being primarily an offshore species, meaning that they live further away from sources of contamination than other predators (Whitehead 2018).
Nielsen et al. (2000) reported levels of cadmium and mercury in the blood of sperm whales stranded in the North Sea at 2,421 and 31,100 μg/l respectively. These levels are high enough to be associated with severe toxicity in other mammal species, although population level effects of such contaminants are unknown (Taylor et al. 2008).
A number of high-profile stranding events of sperm whales have been reported in recent years. A commonality shared by many is the presence of large quantities of plastic, particularly single use plastic bags, in their stomachs (Unger et al. 2016). It is thought that marine debris such as plastic bags are easily mistaken for cephalopods and ingested during feeding dives. Plastic pollution when ingested will block the digestive tract and many animals killed in this way appear emaciated with poor body condition. Plastic ingestion may also cause infection and disease, leading to premature death (Lambertsen 1997).
Climate Change
In response to climate change, it is unknown what the long-term outlook will be for sperm whales. As they are a cosmopolitan species, their range may not be negatively affected (MacLeod 2009). In fact, as they currently range up to the ice-edge, receding sea-ice levels may lead to a range expansion for this species, allowing them to exploit new feeding grounds.
However, how it will affect this species’ response to environmental cues if weather patterns get more unpredictable are not known. With the increased frequency of phenomena such as El Niño, the food supply of sperm whales may also become more unpredictable.
Research in NAMMCO member countries
Although they have not been the target species, sperm whales have been observed and recorded on multiple sightings surveys undertaken by NAMMCO member countries in the North Atlantic. They are, for example, included in the series of North Atlantic Sighting Surveys (NASS) and their abundance has been estimated for the waters around the Faroe Islands, Greenland, Iceland and Norway.
Norway
Research is also being conducted on sperm whales off the Vesterålen Islands in Northern Norway. This includes photo identification done in collaboration with whale watching vessels operating from Andenes and Stø during the summer, which contributes to a dataset that first began in 1989. These identification images have been used to estimate both the number and residency patterns of sperm whales in the area (Lettevall 2003, Zanoni 2004, Rødland 2011). This research has shown a change in the distribution pattern, with sperm whales no longer concentrated in the Bleik Canyon but increasingly dispersed both further offshore and in the shallow waters in Andfjord.
Part of the new summer feeding grounds for male sperm whales documented in Vesterålen overlaps with the coastal long line fishery for Greenland halibut (Reinhardtius hippoglossoides). Episodes of depredation (sperm whales eating halibut off the lines) have been observed and pilot studies on this behaviour were conducted in 2016 and 2017.
A newly established research project led by Tiu Similä – the “Arctic Sperm Whale Project” – will study the behavioural ecology of male sperm whales in the waters off Vesterålen and Senja in more depth in the years to come. This will include collaboration with French researchers studying depredation behaviour of sperm whales and killer whales in the Kerguelen and Crozet islands using satellite transmitters that provide data on acceleration in addition to position and depth. This research will help develop a better understanding of movement, habitat use, and depredation by sperm whales in the North Atlantic.
In 2020, the Norwegian Polar Institute and the Arctic University of Norway placed satellite tags on 12 sperm whales off the coast of Northern Norway and Svalbard. Biopsies were collected from 5 of these individuals for various studies (National Progress Report Norway 2020).
Alexander, A., Steel, D., Hoekzema, K., Mesnick, S.L., Engelhaupt, D., Kerr, I., Payne, R. and Baker, C.S. (2016). What influences the worldwide genetic structure of sperm whales (Physeter macrocephalus)? Molecular Ecology, 25, 2754-2772. https://doi.org/10.1111/mec.13638
Barlow, J. and Cameron, G.A. (2003). Field experiments show that acoustic pingers reduce marine mammal bycatch in the California drift gill net fishery. Marine Mammal Science, 19, 265-283. https://doi.org/10.1111/j.1748-7692.2003.tb01108.x
Baskaran, N., Kanakasabai, R. and Desai, A.A. (2018). Ranging and Spacing Behaviour of Asian Elephant (Elephas maximus Linnaeus) in the Tropical Forests of Southern India. In Sivaperuman, C. and Venkataraman, C. (eds.), Indian Hotspots (p. 295-315). Singapore: Springer. https://doi.org/10.1007/978-981-10-6605-4_15
Begg, G.A. and Waldman, J.R. (1999). An holistic approach to fish stock identification. Fisheries Research, 43, 35-44. https://doi.org/10.1016/S0165-7836(99)00065-X
Berzin, A.A. (1971). The Sperm Whale (edited by Yablokov A.V.).
Best, P.B. (1969). The Sperm Whale (Physeter Catodon) Off the West Coast of South Africa 4. Distribution and movements.
Chiquet, R.A., Ma, B., Ackleh, A.S., Pal, N. and Sidorovskaia N. (2013). Demographic analysis of sperm whales using matrix population models. Ecological Modelling, 248, 71–79. https://doi.org/10.1016/j.ecolmodel.2012.09.023
Clapham, P. (1997). Whales. Colin Baxter Photography Ltd.
Clarke, M.R. (1980). Cephalapoda in the diet of sperm whales of the southern hemisphere and their bearing on sperm whale biology. Discovery Reports, 37, 1-324.
Clarke, R., Aguayo, A. and Del Campo, S.B. (1978). Whale Observation and Whale Marking Off the Coast of Chile in 1964. Scientific Reports of the Whales Research Institute Tokyo, 3, 117-178.
Cetacean offshore distribution and abundance in the European Atlantic (CODA). (2009). Appendix II. Model-based estimates of cetacean abundance in offshore European Atlantic waters.
Eisenberg, J.F., McKay, G.M. and Jainudeen, M.R. (1971). Reproductive Behavior of the Asiatic Elephant (Elephas maximus maximus L.). Behaviour, 38, 193–225. https://doi.org/10.1163/156853971X00087
Felix, F., Haase, B., Davis, J.W., Chiluiza, D. and Amador, P. (1997). A note on recent strandings and bycatches of sperm whales (Physeter macrocephalus) and humpback whales (Megaptera novaeangliae) in Ecuador. Reports of the International Whaling Commission, 47, 917–919
Gero, S., Bøttcher, A., Whitehead, H. and Madsen, P.T. (2016). Socially segregated, sympatric sperm whale clans in the Atlantic Ocean. Royal Society Open Science, 3(6). https://doi.org/10.1098/rsos.160061
Gunnlaugsson, T., Víkingsson, G.A. and Pike, D.G. (2009). Combined line-transect and cue-count estimate of sperm whale abundance in the North Atlantic, from Icelandic NASS-2001 shipboard survey. NAMMCO Scientific Publications, 7, 73–80. https://doi.org/10.7557/3.2706
Hamer, D.J., Childerhouse, S.J., Gales, N.J. (2012). Odontocete bycatch and depredation in longline fisheries: A review of available literature and of potential solutions. Marine Mammal Science, 28, E345-E374. https://doi.org/10.1111/j.1748-7692.2011.00544.x
Harris, C.M., Sadykova. D., Deruiter, S.L., Tyack, P.L., Miller, P.J.O., Kvadsheim, P.H., Lam, F.P.A., Thomas, A.L., Harris, C. and Peters, D.P.C. (2015). Dose response severity functions for acoustic disturbance in cetaceans using recurrent event survival analysis. Ecosphere, 6(11), 1-14. https://doi.org/10.1890/ES15-00242.1
Jaquet, N. (1996). How spatial and temporal scales influence understanding of Sperm Whale distribution: A review. Mammal Review, 26, 51–65. https://doi.org/10.1111/j.1365-2907.1996.tb00146.x
Jefferson, T.A., Webber, M.A. and Pitman, R.L. (2008). Marine mammals of the world : a comprehensive guide to their identification. Academic
Laist, D.W., Knowlton, A.R., Mead, J.G., Collet, A.S. and Podesta, M. (2001). Collisions between ships and whales. Marine Mammal Science. Marine Mammal Science, 17, 35-75. https://doi.org/10.1111/j.1748-7692.2001.tb00980.x
Lambertsen, R.H. (1997). Natural disease problems of the sperm whale. Bulletin van het Koninklijk Belgisch Instituut voor natuurwetenschappen, 67, 105–112.
Lanfredi, C. 2023. Physeter macrocephalus (Europe assessment). The IUCN Red List of Threatened Species 2023: e.T41755A218568250. Accessed on 22 December 2023.
Lettevall, E. (2003). Abundance, association and movement within discrete populations of sperm whales, Physeter macrocephalus. Ph.D. dissertation. Göteborg University, Sweden.
MacLeod, C.D. (2009). Global climate change, range changes and potential implications for the conservation of marine cetaceans: A review and synthesis. Endangered Species Research, 7, 125–136. https://doi.org/10.3354/esr00197
Madsen, P.T., Wahlberg, M. and Møhl, B. (2002). Male sperm whale (Physeter macrocephalus) acoustics in a high-latitude habitat: Implications for echolocation and communication. Behavioral Ecology and Sociobioly, 53, 31-41. https://doi.org/10.1007/s00265-002-0548-1
Martin, A. and Clarke, M.R. (2019). The Diet of Sperm Whales ( Physeter Macrocephalus) Captured Between Iceland and Greenland. Journal of the Marine Biological Association of the United Kingdom, 66, 779–790. https://doi.org/10.1017/S0025315400048426
North Atlantic Marine Mammal Commission (NAMMCO). (2018). Report of the 25th Scientific Committee Meeting, 13-16 November, MS Polarlys, Norway. https://nammco.no/topics/scientific-committee-reports/
Nielsen, J.B., Nielsen, F., Jørgensen, P.-J. and Grandjean, P. (2000). Toxic metals and selenium in blood from pilot whales ans sperm whales. Marine Pollution Bulletin, 40, 348-351. https://doi.org/10.1016/S0025-326X(99)00231-3
Norris, K.S. and Harvey, G.W. (1972). Theory for the Function of the Spermaceti Organ of the Sperm Whale (Physeter catodon L.). NASA Special Publications, 262, Animal Orientation and Navigation edition, 397-417.
Notarbartolo-Di-Sciara, G. (2014). Sperm whales, Physeter macrocephalus, in the Mediterranean Sea: A summary of status, threats, and conservation recommendations. Aquatic Conservation Marine and Freshwater Ecosystems, 24, 4-10. https://doi.org/10.1002/aqc.2409
Pike, D.G., Gunnlaugsson, T., Mikkelsen, B. and Víkingsson, G. (2019). Estimates of the abundance of cetaceans from the NASS Icelandic and Faroese ship surveys conducted in 2015. NAMMCO Scientific Publications, 11. https://doi.org/10.7557/3.4941
Pitman, R.L., Ballance, L.T., Mesnick, S.I. and Chivers, S.J. (2001). Killer whale predation on sperm whales: Observations and implications. Marine Mammal Science, 17, 494-507. https://doi.org/10.1111/j.1748-7692.2001.tb01000.x
Rendell, L. and Whitehead, H. (2005). Spatial and temporal variation in sperm whale coda vocalizations: Stable usage and local dialects. Animal Behaviour, 70, 191-198. https://doi.org/10.1016/j.anbehav.2005.03.001
Rogan, E., Cañadas, A., Macleod, K., Santos, M.B., Mikkelsen, B., Uriarte, A., Van Canneyt, O., Vázquez, J.A. and Hammond, P.S. (2017). Distribution, abundance and habitat use of deep diving cetaceans in the North-East Atlantic. Deep Sea Research Part II: Tropical Studies Oceanography, 141, 8-19. https://doi.org/10.1016/j.dsr2.2017.03.015
Rødland, E.S. (2011). Population estimation of sperm whales (Physeter microcephalus) from Bleik Canyon, Norway. MSc thesis, University of Oslo, Norway.
SCANS-III. (2017). Estimates of cetacean abundance in European Atlantic waters in summer 2016 from the SCANS-III aerial and shipboard surveys Authors and Partners: Additional project personnel: Equipment development.
Schrey, A.W. and Heist, E. (2003). Microsatellite analysis of population structure in the shortfin mako (Isurus oxyrinchus). Canadian Journal of Fisheries and Aquatic Science, 60(6), 670-675. https://doi.org/10.1139/f03-064
Shirihai, H. and Jarrett, B. (2006). Whales, dolphins, and seals : a field guide to the marine mammals of the world. A. & C. Black
Smith, S.C. and Whitehead, H. (2000). The diet of Galápagos sperm whales by fecal sample analysis. Marine Mammal Science, 16, 315-325. https://doi.org/10.1111/j.1748-7692.2000.tb00927.x
Taylor, B.L., Baird, R., Barlow, J., Dawson, S.M., Ford, J., Mead, J.G., Notarbartolo-di-Sciara, G., Wade, P. and Pitman, R.L. (2008). Physeter macrocephalus. The IUCN Red List of Threatened Species. Version 2014.3. IUCN Red List Threat Species Version 20143 8235
Teloni, V., Mark, J.P., Patrick, M.J. and Peter, M.T. (2007). Shallow food for deep divers: Dynamic foraging behavior of male sperm whales in a high latitude habitat. Journal of Experimental Marine Biology and Ecology, 354, 119-131. https://doi.org/10.1016/j.jembe.2007.10.010
Unger, B., Bravo Rebolledo, E.L., Deaville, R., Gröne, A., Ijsseldijk, L.L., Leopold, M.F., Siebert, U., Spitz, J., Wohlsein, P. and Herr, H. (2016). Large amounts of marine debris found in sperm whales stranded along the North Sea coast in early 2016. Marine Pollution Bulletin, 112(1-2), 134–141. https://doi.org/10.1016/j.marpolbul.2016.08.027
WDCS. (2010). Reinventing the Whale.
Whitehead, H. (1996) Variation in the Feeding Success of Sperm Whales: Temporal Scale, Spatial Scale and Relationship to Migrations. Journal of Animal Ecology, 65(4), 429-438. https://doi.org/10.2307/5778
Whitehead, H. (2003). Sperm whales : social evolution in the ocean. University of Chicago Press
Whitehead, H. (2018). Sperm Whale: Physeter macrocephalus. Encyclopedia of Marine Mammal (3rd ed.), 919-925. https://doi.org/10.1016/B978-0-12-804327-1.00242-9
Whitehead, H., Christal, J. and Dufault, S. (1997). Past and Distant Whaling and the Rapid Decline of Sperm Whales off the Galápagos Islands. Conservation Biology, 11(6), 1387-1396. https://doi.org/10.1046/j.1523-1739.1997.96246.x
Wilson, D.E. and Reeder, D.M. (2005). Mammal species of the world : a taxonomic and geographic reference. Johns Hopkins University Press.
Wood, M.P. and Carter, L. (2008). Whale entanglements with submarine telecommunication cables. IEEE Journal of Oceanic Engineering, 33(4), 445–450. https://doi.org/10.1109/JOE.2008.2001638
Zimmer, W.M.X., Tyack, P.L., Johnson, M.P. and Madsen ,P.T. (2005). Three-dimensional beam pattern of regular sperm whale clicks confirms bent-horn hypothesis. Journal of the Acoustical Society of America, 117, 1473. https://doi.org/10.1121/1.1828501
Øien, N. (2009). Distribution and abundance of large whales in Norwegian and adjacent waters based on ship surveys 1995-2001. NAMMCO Scientific Publications, 7, 31-48. https://doi.org/10.7557/3.2704