Harbour Porpoise
Latest update: July 2021
The harbour porpoise is one of the smallest cetaceans in the world. It has a dark grey colour overall, with sides that are lighter grey. A characteristic feature of these porpoises is the presence of tubercles (small hard bumps) on the leading edge of the dorsal fin. Their bodies are robust, with a small triangular dorsal fin located in the middle of the back, and relatively small, pointed flippers. The head is rounded with a poorly demarcated beak. The harbour porpoise is found primarily in coastal areas, and it has a widespread distribution throughout the northern hemisphere. It is very abundant, with a global population numbering at least 700,000 (Hammond et al. 2008).
ABUNDANCE
The harbour porpoise is very abundant, with a global population numbering at least 700,000 (Hammond et al. 2008).
DISTRIBUTION
The species is primarily found in coastal areas.
RELATION TO HUMANS
Harbour porpoises have historically been hunted for meat and blubber. In the NAMMCO area it is currently hunted only in Greenland, with a yearly catch of around 2,500.
CONSERVATION AND MANAGEMENT
Catch numbers are reported annually by the Greenlandic government. In some of its major habitats, conservation measures have been implemented following concerns about levels of by-catch in commercial fisheries.
In the most recent regional European assessment, the species is listed as ‘Least Concern’ on the IUCN Red List (2023) The species listed as ‘Least Concern’ in both Norwegian and Icelandic national red lists.
LIFESPAN
Less than 5% of the population live beyond 12 years, but the maximum longevity recorded is 24 years.
AVERAGE SIZE
Adults are on average 141-163 cm long and weigh 45-65 kg, with females being larger than males at all ages.
MIGRATION AND MOVEMENTS
While there are noticeable seasonal shifts in distribution in certain locations, these porpoises do not appear to undertake coordinated migrations. Tidal currents appear to influence movements in a number of locations.
FEEDING
Harbour porpoises are flexible and opportunistic in their feeding. Their diet varies by season, year and location. They take a wide variety of prey species from both benthic and pelagic habitats, but porpoises in one area tend to feed primarily on two or three species of fish.
General Characteristics
The harbour porpoise (Phocoena phocoena) is one of the smallest cetaceans in the world, with a widespread distribution throughout the northern hemisphere. It is very abundant, with a current global population numbering around 700,000 (Hammond et al. 2008).
As its name implies, this porpoise is found primarily in coastal areas, though they are sometimes found over deeper offshore waters. They also frequent river estuaries, occasionally moving up the rivers many miles from the sea. The harbour porpoise has an almost circumpolar distribution, with four subspecies recognized in different regions: P. p. phocoena in the North Atlantic, P. p. vomerina, in the eastern North Pacific, an un-named subspecies in the western North Pacific and P. p. relicta in the Black Sea (Hammond et al. 2008). This account will deal with the North Atlantic subspecies: P. p. phocoena.
Appearance, size and longevity
The harbour porpoise has a dark grey colour overall, with sides that are lighter grey. The ventral side is light grey or white, and in most individuals there are grey stripes running from the mouth along the throat to the front of the flippers. The body is robust, with a small triangular dorsal fin located in the middle of the back and relatively small, pointed flippers. The head is rounded with a poorly demarcated beak. The skull contains from 22 to 28 pairs of small spade-shaped teeth in the upper jaw, and 21 to 25 pairs in the lower. A characteristic feature of these porpoises is the presence of tubercles (small hard bumps) on the leading edge of the dorsal fin. It is not known if these have a specific purpose (Bjørge & Tolley 2018).
In the North Atlantic, adults of both sexes attain lengths from 141 to 163 cm and body masses between 46-65 kg, with females being larger than males at all ages (Gaskin 1992, Lockyer 2003). At birth the harbour porpoise averages 65-75 cm long and weighs from 6 to 10 kg. The maximum longevity recorded is 24 years. However, less than 5% of the population live beyond 12 years (Lockyer 2003).
Life History and Ecology
Behaviour
At the surface
Harbour porpoises are a difficult cetacean to detect visually. Their small size and barely visible blow, as well as the fact that they generally spend little time at the surface and do not often engage in visible behaviours such as breaching or tail slapping, make them hard to spot. One study of porpoises feeding off the coast of Wales observed breaching at a rate of approximately once every 120 sightings (Pierpoint 2008). Other behaviours observed in this study were “tail flipping”, where a porpoise would pivot abruptly at the surface, throwing their flukes forwards through the air (once every 240 sightings) and tail slapping (once in 300 sightings).
Diving
Harbour porpoises are not deep divers and generally make short but frequent dives from the surface. One study of tagged porpoises found a maximum dive depth of 34 m and a maximum duration of 213 seconds (Linnenschmidt et al. 2013). There was, however, a large variability in diving activity observed, with porpoises making anywhere from 6 to 179 dives per hour.
Social behaviour
Harbour porpoises are most often seen alone or in small groups of 2-3 animals. In the SCANS aerial surveys (studying the distribution and abundance of cetaceans in European Atlantic waters), the mean group size observed was 1.35, while a mean group size of 1.53 was seen from the ship survey (Hammond et al. 2017). A similar group size (mean of 1.3 animals) was found in the Marsdiep area of The Netherlands, with only a single porpoise seen in 72% of cases (Ijsseldijk et al. 2014). In the central North Sea, a mean group size of 1.6 (SD 1.3) individuals was observed from a ship survey, with groups between 1-6 animals seen at any one time (Cucknell et al. 2017).
Slightly larger groups were observed in a feeding study off the coast of Wales. At one location, groups of up to eight porpoises were commonly seen swimming abreast in a closely-spaced line, surfacing simultaneously and repeatedly. It was not clear, however, whether they were cooperating to feed or had just converged on a school of prey (Pierpoint 2008). Cooperative foraging has not been commonly observed in this species.
Sounds
Like many other toothed whales, harbour porpoises produce short pulsed high frequency (110-180 kHz) “click” sounds, primarily for echolocation (Carlström 2005, Cucknell et al. 2017). The rate of production of these clicks appears to vary with time of day, but also between individuals. Three tagged porpoises in one study had levels of echolocation activity from fewer than 100 to over 50,000 clicks per hour (Linnenschmidt et al. 2013).
Harbour porpoise clicks are distinctive to the species, and do not carry very far under water, perhaps to a maximum of 1000m (Clausen et al. 2011). Clicks may be emitted singly or in a series called “click trains”. When echolocating, the click interval gets shorter the closer the animal is to its target (Todd et al. 2009). The use of pulsed, short-range clicks rather than whistling or other vocalizations has been suggested as an adaptation by these porpoises to avoid detection by killer whales or other predators (Clausen et al. 2011, Kyhn et al. 2013).
Sound activity
Studies on tagged harbour porpoises in different parts of their range have found differences in day and night patterns in click activity, with most studies showing a higher production of clicks at night (e.g. Carlström 2005, Todd et al. 2009, Linnenschmidt et al. 2013). The difference in click activity is thought to be linked to foraging behaviour, which is likely related to the availability of the porpoises’ preferred prey species.
Harbour porpoises also appear to use clicks to communicate with each other. In a study of captive porpoises, specific patterns of clicks were observed that were linked to specific behaviours (Clausen et al. 2011). Aggressive behaviour, for example, was noted in which a porpoise would direct a high rate and volume of clicks at another. Porpoises exposed to this would move away from the animal producing the sounds. The same study did not find any indication that porpoises produce individual “signature” clicks that would allow them to recognize each other.
Strandings
Harbour porpoises are commonly found stranded throughout their range. Along the German North Sea coast, for example, 996 harbour porpoises were found stranded in the period 1990 to 2001, and a further 229 porpoises were found along the German Baltic Sea coast (Siebert et al. 2006). Between 2003 and 2008, an average of just over one harbour porpoise per week (1.23) was found stranded along the Danish coast (Wright et al. 2013). In the Northwest Atlantic, a total of 515 porpoises were found stranded on the Gulf of Maine and Bay of Fundy shorelines in the period 2009 to 2013 (Waring et al. 2016). Harbour porpoises typically strand one at a time, rather than the mass strandings seen in some other cetacean species.
Timing of strandings
Strandings occur year-round, though may be more common at certain times of the year in some areas. In the North Sea, most porpoises are found stranded during the summer months, with the highest numbers found in June, July and August (Hasselmeier et al. 2004). In the Baltic, most strandings are seen a little later, from July through September. Along the Dutch coast, strandings have been most frequent between January and July (Osinga et al. 2008). Along the northeast coast of the USA, more harbour porpoises are found stranded during the winter months than during the summer (Polacheck et al. 1995).
Strandings may occur when porpoises follow a high tide in to coastal waters and become trapped when the tide recedes. Other stranded animals show signs of predation or interactions with fishing gear. Around the Moray Firth, Scotland, 63% of the harbour porpoises found stranded appeared to have been attacked by bottlenose dolphins and died from trauma, specifically bone fractures and internal injuries (Ross and Wilson 1996).
By-catch and entanglements
Interactions with fishing gear are a significant source of mortality for harbour porpoises throughout their range, and animals that die in this way may be found along coastlines. Along the German Baltic Sea coast, by-catch was identified as the cause of death in 47–86% of the annual strandings between 2000 and 2009 (Culika et al. 2015), as well as for about one-third of the porpoises found dead in an “unusual mortality event” in which 85 animals stranded in a single week in Denmark (Wright et al. 2013).

Life History
Age at sexual maturity for harbour porpoises varies by region, but is generally between 3 and 4 years of age for both sexes. It may, however be as young as 2 or as old as 5 years (Lockyer 2003). In the Western North Atlantic, sexual maturity for both sexes is attained between 3 and 4 years old (Gaskin 1992). Most female porpoises in the Gulf of Maine were found to be mature at age 3 (Read and Hohn 1995). In the Eastern North Atlantic, maturity appears to occur later. Female porpoises from the German North Sea and Baltic Sea were found to be mature at an average age of 4.95 years (Kesselring et al. 2017), and in Scottish waters, age at sexual maturity was estimated at 4.35 years for females and 5.00 years for males (Learmonth et al. 2014).
Reproduction
The mating season is in the summer, with mating occurring shortly after birth. In the Bay of Fundy, births occurred from May to July, with most births in May (Gaskin 1992). A similar period (May to July) was observed for porpoises in Scottish waters (Learmonth et al. 2014). In the German North Sea and Baltic seas, the birth period is from June through August (Kesselring et al. 2017). Elsewhere in the North Sea, a birth period of between 6 June and 16 July was determined from examinations of stranded animals, with a mean date of birth of 27 June (Hasselmeier et al. 2004). This is close to the mean birth date of 30 June previously calculated for harbour porpoises in Danish waters (Sørensen and Kinze 1994).
After mating, there appears to be delayed implantation of 6 or 7 weeks (Gaskin 1992), resulting in a gestation period of 10 to 11 months (Lockyer 2003, Learmonth et al. 2014). Most females produce a calf each year. The lactation period is 8 or 9 months, although the calves may start taking solid food at around 5 months old. Calves remain with their mother for a period of around 18 months (Gaskin 1992).
Food and feeding
Studies of harbour porpoise diet have shown that it varies by season and location, and that these porpoises take a wide variety of prey species from both benthic and pelagic habitats. In many areas, small pelagic schooling fish such as herring and mackerel are important in the diet, while in other locations demersal fish such as sandeels predominate. In a study of stomach contents from 247 porpoises by-caught or stranded in the waters of Denmark, Sweden and Norway, a minimum of 30 different species of fish were identified, representing 16 families (Aarefjord et al. 1995). In porpoises from Icelandic waters, more than 40 different fish and invertebrate prey taxa were identified (Víkingsson et al. 2003). Porpoises along the northeast Atlantic coast of France were found to take 13 different species of fish and 5 species of cephalopods (Spitz et al. 2006).
Main prey species
While they may take a wide variety of species, porpoises in any one area tend to feed primarily on two or three species of fish. In the Gulf of St. Lawrence, for example, capelin (Mallotus villosus), Atlantic herring (Clupea harengus), and redfish (Sebastes marinus) were the three most important species in the diet, with capelin and herring making up more than 80% of the mass and caloric contribution to the diet (Fontaine et al. 1994).
Capelin was found to be an important prey species for porpoises off Northern Norway, while herring (Clupea harengus) was the single most important species in the diet overall in Scandinavian waters (Aarefjord et al. 1995). Capelin was also found to be the predominant prey of porpoises in Icelandic waters (Víkingsson et al. 2003). In waters off Scotland, whiting (Merlangius merlangus) and sandeels (Ammodytidae) were the most important species in the diet, where they made up more than 84% of the prey mass (Santos and Peirce 2003, Santos et al. 2004).
Blue whiting (Micromesistius poutassou), was the most frequent prey found in porpoise stomachs from the northeast coast of France, with sardine (Sardina pilchardus), scads (Trachurus sp.) and whiting (Merlangius merlangus) also being important (Spitz et al. 2006). In the western Baltic Sea, Atlantic cod and herring were the main prey of adult porpoises, constituting on average 70% of the diet mass (Andreasen et al. 2017).
Harbour porpoises generally appear to be flexible and opportunistic in their feeding, with variation not only by location, but by year and season. Differences in diet have also been seen between porpoises of differing ages and sexes. These may be due to either selective predation by the porpoises, or changes in the distribution and abundance of the prey.
Predators
Direct evidence of predation on harbour porpoises has been found for the white shark (Carcharodon carcharias), and for killer whales (Orcinus orca) (Arnold 1972, Bjørge and Tolley 2018). Grey seals (Halichoerus grypus) have also been observed both preying on and scavenging carcasses of harbour porpoises off both the coast of France in the eastern English Channel and the coast of Wales (Bouveroux et al. 2014, Stringell et al. 2015).
While perhaps not direct predation, violent and fatal attacks on harbour porpoises by bottlenose dolphins have been reported in several locations (Santos and Peirce 2003, Spitz et al. 2006). Around the Moray Firth, Scotland, 63% of harbour porpoises stranded were found to have died from trauma, particularly multiple bone fractures and damaged internal organs, and many of the dead porpoises had cuts on their skin corresponding to dolphin teeth marks (Ross and Wilson 1996). Violent interactions between dolphins and porpoises were also witnessed directly.
Health – diseases and parasites
Little is known about the role of disease and parasitism in the health and natural mortality of harbour porpoises.
Parasites
Parasites in harbour porpoises have not been well studied. A Danish investigation of 70 stranded porpoises found 5 species of helminths in the digestive tracts: Anisakis simplex, Hysterothylacium aduncum, Pholeter gastrophilus, Bolbosoma sp. and Diphyllobothrium sp. (Herreras et al. 1997), relatively few species compared to other cetaceans. This may be because harbour porpoises tend to be found singly or in very small groups instead of large schools or pods, and because their life span is short relative to other toothed whales.
In another study, stranded porpoises found on the Danish coast had nematodes in the stomach and around the heart, 4 of 85 animals had trematodes in the liver, and 5 had mild to severe parasitic infections in the ear sinuses (Wright et al. 2013). These parasites were not identified to species.
In West Greenland, 20 harbour porpoises were investigated for parasites. Protozoa (Sarcocystis sp.), Nematoda (Halocercus invaginatus, Stenurus minor, Anisakis simplex sensu lato (s.l.), Crassicauda sp.), Trematoda (Campula oblonga) and Cestoda (Phyllobothrium delphini, Monorygma grimaldii) were found (Lehnert et al. 2014). The nematode H. invaginatus was the most common parasite, and was found in the lungs of 19 out of 20 porpoises, while Campula oblonga was found in the pancreas and liver of 18 animals. Health effects of these parasites on the porpoises were bronchopneumonia, gastritis, cholangitis, pancreatitis and panniculitis, though these were usually mild (Lehnert et al. 2014).
Starvation
While not a disease, harbour porpoises are prone to starvation. Because of their small size and limited energy reserves, they must feed frequently in order to maintain good body condition. If their food source is unavailable, they may die. Juveniles may be especially prone to starvation. Each spring, many emaciated juvenile porpoises are found stranded along the northeastern U.S. east coast, apparently starved to death (COSEWIC 2006). Emaciation was also noted as a common cause of death in porpoises found stranded on the Dutch coast (Osinga et al. 2008).
Distribution and habitat
The harbour porpoise is found primarily in coastal areas, though they may sometimes travel over deeper offshore waters. They also frequent river estuaries, occasionally moving up the rivers many miles from the sea.
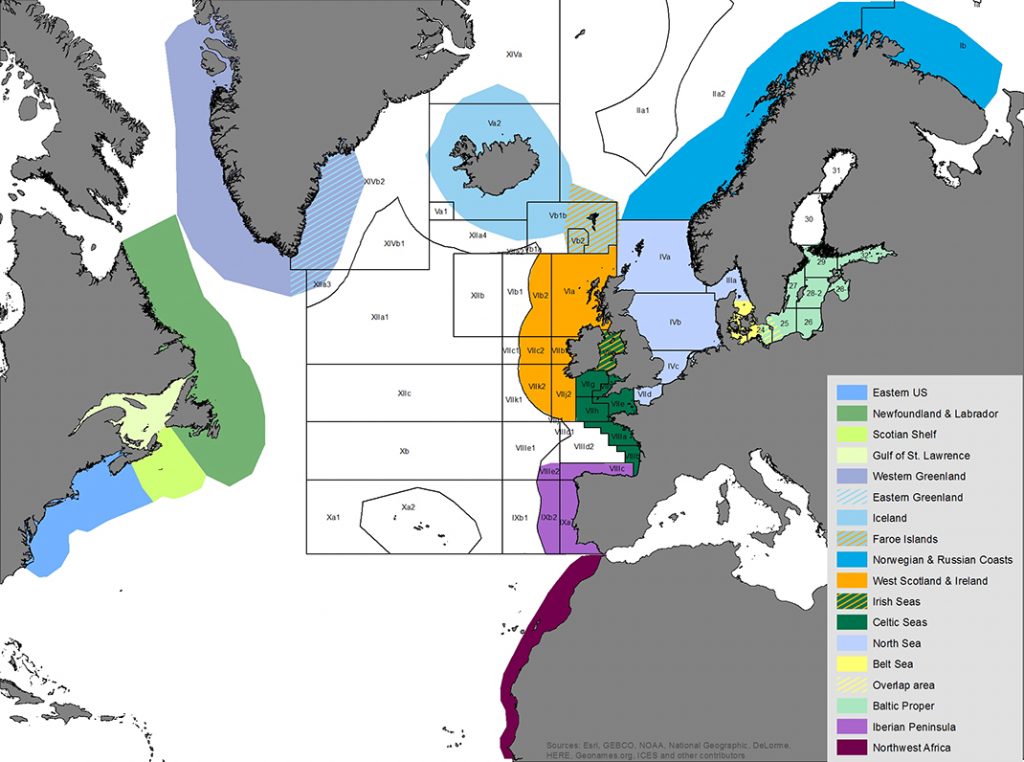
Map of the assessment areas for harbour porpoises in the North Atlantic as used during the International Workshop on the Status of Harbour Porpoises in the North Atlantic (NAMMCO & IMR 2019). ICES fishing areas super-imposed and unclear divisions between assessment areas indicated by hatched colours. Cartography: Solveig Enoksen
Northwest Atlantic
In the western North Atlantic, harbour porpoises are found along the United States coast as far south as northern Florida, but more commonly down to Cape Hatteras, North Carolina (Donovan & Bjørge 1995), and north along the Newfoundland and Labrador coasts to southern Baffin Island.
Northeast Atlantic
In the eastern North Atlantic, harbour porpoises are common and widely distributed over the continental shelf and are found primarily at depths of 20-200 m. They range as far south as Senegal in Northwest Africa and as far north as Novaya Zemlya and the Barents Sea. These porpoises also occur around Greenland (as far north as Upernavik on the west coast), as well as in the waters around Iceland and the Faroe Islands. They also inhabit the waters around the UK and Ireland, and the North Sea. The harbour porpoise is also the only cetacean currently found in the Baltic Sea.
In the North Sea, there have been distinct shifts in harbour porpoise distribution over the past decades. After becoming “virtually extinct” in Dutch coastal waters during the 1960s (Camphuysen 2004), they are now present along that coast and throughout the southern North Sea in increasing numbers (Alfonsi et al. 2012, Peschko et al. 2016). The multinational Small Cetacean Abundance in the North Sea and Adjacent waters (SCANS) surveys, which have been carried out in 1994, 2005 and again in 2016, documented this trend with an overall shift in harbour porpoise distribution from north to south and into the English Channel (Hammond et al. 2017).

Migrations and movements
While there are noticeable seasonal shifts in harbour porpoise distribution in certain locations, these porpoises do not appear to undertake coordinated migrations.
Satellite telemetry
Satellite telemetry to track harbour porpoise movements has been used in various studies throughout their range. In the Bay of Fundy and Gulf of Maine, tagged porpoises were seen to remain in one location for periods of days to weeks before moving rapidly to another location (Read & Westgate 1997). In a long term study in the North Sea and western Baltic Sea, tagged harbour porpoises were found to congregate in nine high-density areas rather than being evenly distributed throughout the region (Sveegaard et al. 2011). The same study found that immature porpoises travelled over larger areas than mature porpoises did.
Two female porpoises, one adult and one subadult, were tagged in West Greenland in 2012. The tags provided positions for more than a year. Both animals made extensive movements around Greenland, the central North Atlantic, and into Canadian waters. One animal wintered in the central North Atlantic, the other in offshore waters between Greenland and Canada. Both animals returned to the tagging site in West Greenland the following summer. They travelled over 17,500 km and 10,000 km, respectively, and spent on average 83% of their time in offshore areas with depths of more than 200 m (Nielsen et al. 2013). These findings suggest that harbour porpoises may utilize offshore waters to a greater extent than was previously thought.
Seasonal movements
Porpoises have been seen to make seasonal movements, for example moving out of the western part of the German Baltic Sea in wintertime (Verfuß et al. 2006), but these appeared to be more individual and gradual movements rather than a synchronized migration. In the southern parts of the North Sea, harbour porpoise densities in Dutch and German coastal waters peak in the winter and spring, and decline through the summer and fall, suggesting offshore movements during these seasons.
A similar pattern is seen along the Belgian coast (Haelters et al. 2011). Observations along the Dutch coast suggest that porpoises are moving inshore and offshore, rather than in a north-south direction (Camphuysen 2004). In the central North Sea, however, porpoises were found to be present year-round (Cucknell et al. 2017). In the more northern parts of their range, harbour porpoises may move to offshore waters in the winter to avoid nearshore ice (Bjørge & Tolley 2018).
Tidal currents appear to influence porpoise movements in a number of locations. Off the coast of Wales, harbour porpoises were observed regularly arriving and departing from a feeding area by moving with the prevailing tide (Pierpoint 2008). Similar behaviour has been seen along the coast of The Netherlands and in the Bay of Fundy (IJsseldijk et al. 2015, Johnston et al. 2005).
North Atlantic stocks
In the North Atlantic, 13 different populations or sub-populations of harbour porpoises have been proposed, with a 14th group inhabiting the Black Sea (Donovan and Bjørge 1995, Andersen 2003). Even though there is a clear distinction based on genetic analysis, the group structure within the Northwest and Northeast Atlantic is not clear. Part of the reason for this is that not all areas have been equally studied, and sample sizes for some of the suggested sub-populations are low. Differences in behaviour between the sexes also make separating stocks difficult: female porpoises tend to return to the same breeding areas each year while males may travel between areas (Andersen et al. 1997). Recent shifts in overall harbour porpoise distribution, primarily in the North Sea and along the western coasts of Europe are also confusing the population structure in this region.
Genetic investigations
The further apart the populations are geographically, the greater the genetic differences. Adjacent groups, however, appear to be more similar. Genetic analysis of the population structure of harbour porpoises from West Greenland, the North Sea and inner Danish waters, for example, found three differentiated populations but also that there was some degree of gene flow between them (Andersen et al. 1997). Along the coast of France, genetic investigation of porpoises stranded or by-caught between 2000 and 2010 found them to be admixed individuals from the two genetically distinct populations previously identified from the Iberian coasts and further north in the Northeast Atlantic (Alfonsi et al. 2012).
It is also not entirely clear whether the Baltic Sea harbour porpoises are a separate population. One study found that the degree of genetic divergence among harbour porpoises between the Baltic Sea and the Kattegat-Skagerrak waters of Denmark is low (Palme et al. 2008), suggesting a single population in the region instead of two. Recent genetic analysis presented at the Joint IMR/NAMMCO International Workshop on the Status of Harbour Porpoises in the North Atlantic (NAMMCO and IMR 2019) suggested a clear distinction between North Sea and Baltic Sea populations, with a transition zone in the Kattegat. Within the Baltic Sea, a further subdivision was also seen between the western Belt Sea and an eastern inner Baltic sea sub-population, with a transition zone around the southern tip of Sweden. Given that the distinction was most prominent during times of reproduction, some seasonal migration across population boundaries may be occurring. Studies combining genetic analysis, morphometrics, telemetry and passive acoustic monitoring also suggest three distinct populations in the North Sea, the Belt Sea (and adjacent waters) and the Baltic proper (NAMMCO & IMR 2019) .
Recent work
In 2018, a Joint Institute of Marine Research/NAMMCO International Workshop on the Status of Harbour Porpoises in the North Atlantic was held in Tromsø, Norway. This workshop was followed by a working group focusing on harbour porpoises in West Greenland. The reports from both these meetings are available here.
Current abundance and trends
Harbour porpoises are one of the more difficult cetaceans to obtain population estimates for. Their small size and lack of highly visible behaviours at the surface make them difficult to spot, and so the standard methods of aerial or ship surveys are likely to underestimate harbour porpoise numbers unless the survey methods are optimized for this particular species. Furthermore, in some areas, such as Greenland and Newfoundland, they are found throughout many small bays and inlets, which makes surveying additionally difficult. Some surveys have, however, been carried out in parts of their range and some abundance estimates made.
Altogether, it has been estimated that there are at least 700,000 harbour porpoises globally (Hammond et al. 2008).
UK waters and the North Sea
In the North Sea and waters around the UK, a series of surveys known as SCANS (Small Cetacean Abundance in European Atlantic Waters and the North Sea), have been carried out. The first SCANS survey was in 1994, followed by SCANS II in 2005 and SCANS III in 2016. These surveys are large-scale ship and aerial surveys.
From the SCANS III surveys, carried out in July 2016, it was estimated that there were 466,569 harbour porpoises in the study area (95% CI: 345,306 -630,417) (Hammond et al. 2017). Results from SCANS II (2005) are 519,864 (95% CI: 343,521 – 786,730) and for the 1994 SCANS I survey 407,177 (95% CI: 288,920 – 573,838) (Hammond et al. 2017).
One of the first surveys of the North Sea done in winter found an estimated abundance of 62,265 (95% CI: 26,114 – 149,455) harbour porpoises in the central North Sea (Cucknell et al. 2017). The density estimate of 0.63 individuals/km from this survey is very similar to that found in the same general region from the SCANS II summer survey (0.6 individuals/km), suggesting that harbour porpoises inhabit this area of the North Sea year-round.
The Baltic Sea
In the Baltic Sea, a 1995 survey gave an abundance estimate of about 600 harbour porpoises (CV = 0·57), although the entire range in the Baltic was not covered (unpublished data reported in Hammond et al. 2002).
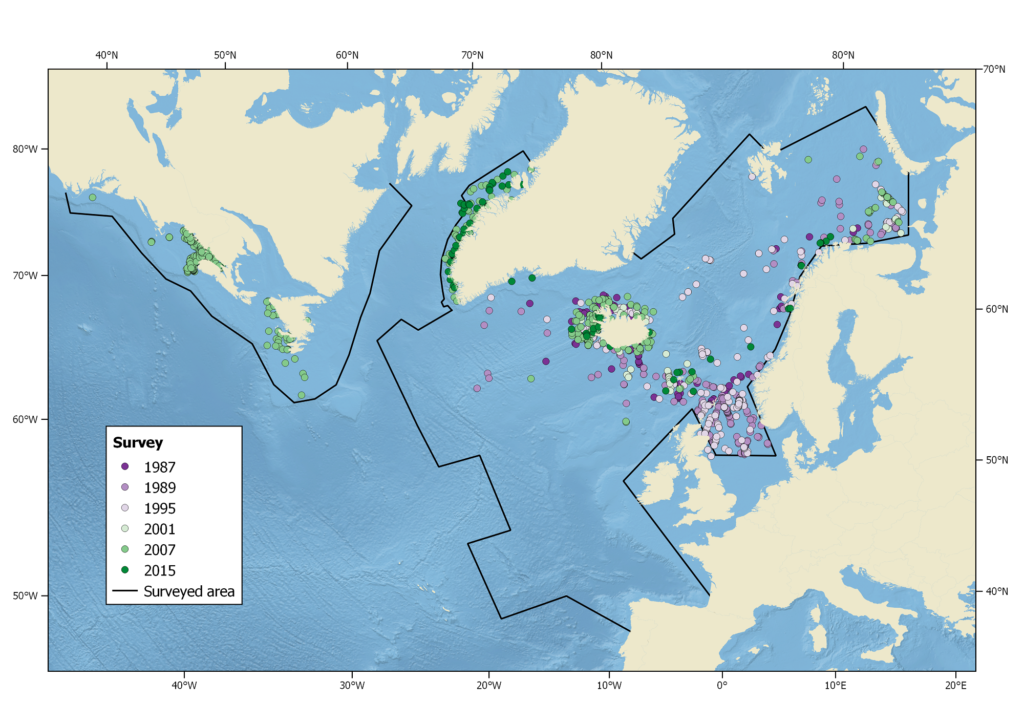
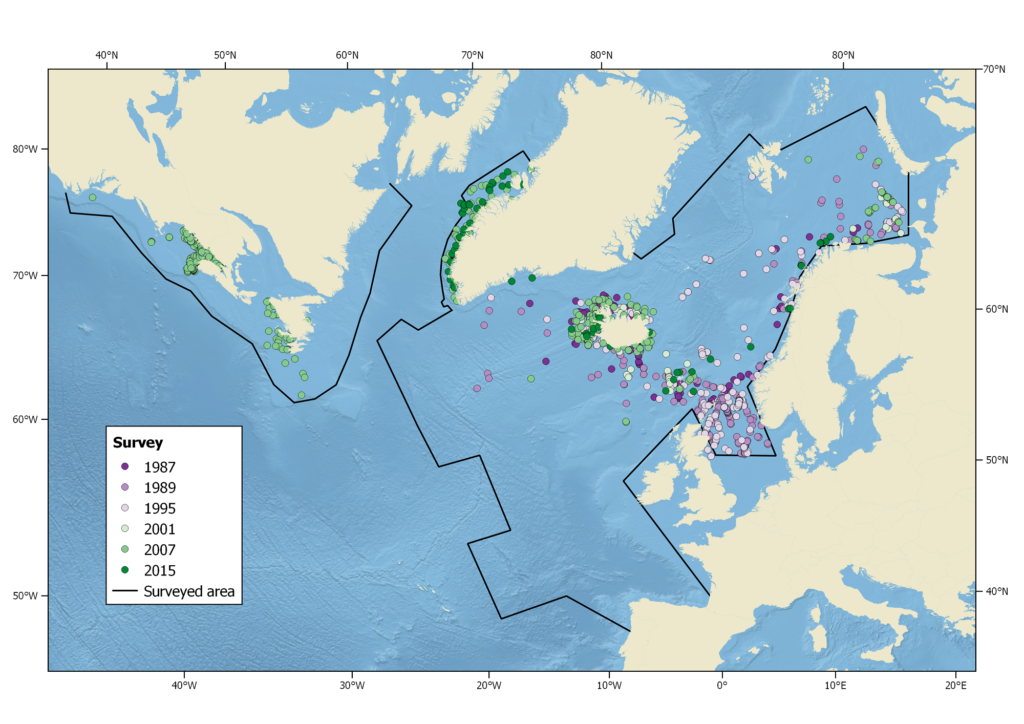
Harbour porpoise sightings during the North Atlantic Sightings Surveys (NASS) from 1987-2015. Not all areas were surveyed in each year.
Norwegian waters
Harbour porpoises were first surveyed in Norwegian waters in 1989. For southern Norway and the northern North Sea, an abundance estimate of 82,600 (95% CI: 52,100 – 131,000) was made at that time, and for Northern Norwegian waters and the Barents Sea the estimate was 11,000 porpoises (95% CI: 4,790 – 25,200) (Bjørge and Øien 1995). From the SCANS III survey, an estimate of 25,000 porpoises was made for the Norwegian coast between Stadt and Vestfjorden (NAMMCO 2017).
After 1995, Norway began a mosaic survey program and from 2003 this was combined with a joint Norwegian-Russian ecosystem survey in which the transits between stations in the joint survey were used as transects for marine mammal detection (NAMMCO 2018). These mosaic surveys cover southern Norway, the northern North Sea and the Barents Sea.
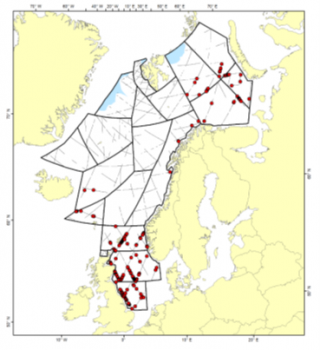
Distributions of sightings of harbour porpoises during the 2014-2018 Norwegian mosaic survey
From the sighting surveys conducted during the period 2002-2007, harbour porpoise abundance was estimated to be 189,604 (95% CI: 129,437 – 277,738). Following the sighting surveys conducted during the period 2008-2013, abundance was estimated at 30,556 (95% CI: 10,178 – 76,120). From surveys conducted during 2014-2018, the estimated abundance was 255,929 animals (95% CI: 172,742 – 379,175). Descriptions of the calculation of each of these abundance estimates, as well as a discussion of the anomalously low 2008-2013 estimate can be found in the 2019 report of the NAMMCO Abundance Estimates Working Group (NAMMCO 2019).
Iceland
Harbour porpoises have been included in cetacean aerial surveys around Iceland, although they are not generally the target species.
Pike et al. (2009) estimated harbour porpoise abundance around Iceland for all North Atlantic Sighting Surveys (NASS) aerial surveys up to 2001. Abundance was estimated to be 4,239 (95% CI: 2,724 – 6,599) in 1986 and 5,156 (95% CI: 3,027 – 8,783) in 1995, while sightings were too few in 1987 and 2001 to develop estimates. The authors acknowledged that abundance was likely substantially underestimated due to uncorrected perception and availability biases.
Gilles et al. (2011) used correction factors developed during the SCANS-II survey, in which the primary harbour porpoise observer had taken part, to develop a fully corrected estimate of 43,179 (95% CI: 31,755 -161,899) for the area in 2007.
The most recent NASS survey was done in 2016. During the 2016 survey, block 5 of the area was not able to be surveyed. Total abundance of harbour porpoises from the 2016 survey was estimated to be 22,806 (95% CI: 9,166 – 56,746) (NAMMCO 2019). This estimate has been corrected for perception bias but not availability bias (i.e. animals that are missed because they are submerged and unavailable for counting) and it should be noted that this bias can be substantial in aerial surveys for this species.
Greenland
In West Greenland, an aerial survey for three whale species, including harbour porpoises, was conducted in the fall of 2007 over the shelf area from 69°N to 59°N. Prior to this study, only limited information was available regarding the distribution and abundance of harbour porpoises in Greenlandic waters. A more recent survey was conducted in 2015 in both West and East Greenlandic waters. The initial calculated total abundance estimate from the 2007 was considered to be an underestimate due to a low number of sightings at the surface and it was revised at the NAMMCO Harbour Porpoise Working Group meeting in November 2013 to include porpoises seen down to 1 m below the surface.
During a meeting of the Harbour Porpoise Working Group in 2019, the estimates for West Greenland from both the 2007 and 2015 surveys were corrected to take into account animals that may not have been present in the strata at the time of the survey. Satellite tracking data from 26 harbour porpoises in Greenland was used to estimate the proportion of porpoises expected to be outside the strata at the time of the survey. On this basis, the fully corrected abundance estimates were 69,595 (95% CI: 34,689 – 139,624) porpoises in 2007, and 106,822 (95% CI: 55,149 – 206,909) porpoises for 2015.
Northwest Atlantic
In the Northwest Atlantic, an abundance estimate of 12,732 (CV=0.61) harbor porpoises on the Scotian Shelf and in the Gulf of St. Lawrence was generated from the Canadian Trans-North Atlantic Sighting Survey in summer 2007 (Lawson & Gosselin 2009). This aerial survey covered the Canadian coast from northern Labrador to the Scotian Shelf. The total estimate including porpoises from the Gulf of Maine and Bay of Fundy was 16,058 (CV=0.50) (NOAA 2016). A more recent survey in the Gulf of Maine and lower Bay of Fundy which utilized both aircraft and surface vessels was carried out between June and August 2011, and generated an abundance estimate of 79,883 (CV=0.32) (NOAA 2016).
Stock status
UK, the North Sea and European Waters
Perhaps the best indication of population status and trends can be obtained from the SCANS series of surveys, which have been carried out in the North Sea and waters around the UK. The first SCANS survey was in 1994, followed by SCANS II in 2005 and SCANS III in 2016. These surveys are large-scale ship and aerial surveys to study the distribution and abundance of harbour porpoises and other cetaceans in European Atlantic waters and the North Sea.
The series of three SCANS surveys do not reveal any trend in the total harbour porpoise population since the mid 1990s (see Current Abundance section). What has been noted, however, is a shift in distribution of porpoises, from the northern to the southern parts of the North Sea, into the English Channel and along the coasts of Belgium and The Netherlands (Camphuysen 2004, Peschko et al. 2016). The reasons for this shift are unclear.
Baltic Sea
One area where a decline in the harbour porpoise population has been noted is in the Baltic Sea. Porpoises were formerly common in the Baltic, but over the past several decades the population has decreased in both distribution and abundance (Hammond et al. 2002). The reason for this is not known, and further research is needed to provide a more complete picture of the harbour porpoise population in the Baltic Sea.
Greenland
The NAMMCO Harbour Porpoise Working Group carried out an assessment for West Greenland in 2019 (NAMMCO 2019b). Bayesian population models were run using the corrected abundance estimates from the 2007 and 2015 surveys (see Abundance section), together with catch and age-structure data. This modelling showed that the population in West Greenland is relatively stable. The report with the the full assessment is available here.
Elsewhere
In other parts of their range, harbour porpoises have not been surveyed systematically enough to establish any population trends.
Management
The harbour porpoise is a widespread and abundant species. It is listed in the International Union for the Conservation of Nature (IUCN) red list in the category of “Least Concern”, although the Baltic Sea sub-population is considered “Critically Endangered” due to low population numbers (Hammond et al. 2008).
While not considered endangered, harbour porpoises are listed in Appendix II of CITES, the Convention on International Trade in Endangered Species of Wild Flora and Fauna, as well as in Appendix II of CMS, the Convention on the Conservation of Migratory Species of Wild Animals. These conventions provide for international cooperation in the management of species that are widely distributed. A further agreement was entered into under the auspices of the CMS, the Agreement on the Conservation of Small Cetaceans of the Baltic, North East Atlantic, Irish and North Seas (ASCOBANS). The ASCOBANS agreement ensures international cooperation for the management of small cetaceans, such as harbour porpoise, in European waters.
Hunting
Although there is some hunt of harbour porpoises, it remains a widespread and abundant species. Hunting is allowed in Greenland year-round, and while there are no quotas or catch limits, catch numbers are reported and monitored annually by the Greenlandic government. Further information also provided under Hunting and Utilisation.
By-catch
In some of the major habitats for harbour porpoises (particularly the shelf waters of the USA and Europe), concerns about levels of by-catch in commercial fisheries have led to conservation measures being implemented. Examples of such measures are limiting seasons or areas for commercial fishing, or the use of acoustic deterrent devices (‘pingers’) on fishing gear. For more information see Other Human Impacts.
Hunting and utilisation
Historically
Harbour porpoises were historically hunted for their meat and blubber across many parts of their range. Large catches were made in the Gulf of Maine and Bay of Fundy in the 18th and 19th centuries. In Denmark, Poland and some of the Baltic countries, harbour porpoises were hunted up until World War II. There was also some hunt in the Faroe Islands, but historically this has been at quite low levels. During the late 1980s, between 10 and 20 harbour porpoises were hunted each year in the Faroe Islands (Larsen 1995 in Stenson 2003), and the most recent report is that 3 were hunted in 1996.
Recent catches
Harbour porpoises are currently only hunted in Greenland, where the total reported catch from 1900 to 2013 was 75,180. The period from 1993 – 2013 saw a significant increase in the annual harbour porpoise hunt due to increased effort, with a total of 45,072 porpoises taken during that time. In this period, almost all the hunt occurred in West Greenland with an average annual catch of 2,139, while only around 13 were taken annually in East Greenland (Nielsen and Heide-Jørgensen 2013). The current catch level is around 2,500 porpoises per year (NAMMCO 2015).
Catches in NAMMCO member countries since 1992
| Country | Species (common name) | Species (scientific name) | Year or Season | Area or Stock | Catch Total | Quota (if applicable) |
|---|---|---|---|---|---|---|
| Faroe Islands | Harbour Porpoise | Phocoena phocoena | 2019-2023 | Faroe Islands | N/A | |
| Faroe Islands | Harbour Porpoise | Phocoena phocoena | 2018 | Faroe Islands | N/A | |
| Faroe Islands | Harbour Porpoise | Phocoena phocoena | 1997-2017 | Faroe Islands | N/A | |
| Faroe Islands | Harbour Porpoise | Phocoena phocoena | 1996 | Faroe Islands | 3 | |
| Faroe Islands | Harbour Porpoise | Phocoena phocoena | 1992-1995 | Faroe Islands | N/A | |
| Greenland | Harbour porpoise | Phocoena phocoena | 2023 | Total | 3619 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2022 | Total | 3199 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2021 | Total | 2969 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2020 | East | 2 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2020 | West | 3329 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2020 | Total | 3331 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2019 | East | 4 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2019 | West | 2774 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2019 | Total | 2778 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2018 | East | 8 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2018 | West | 2882 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2018 | Total | 2890 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2017 | East | 6 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2017 | West | 2379 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2017 | Total | 2385 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2016 | East | 8 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2016 | West | 2372 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2016 | Total | 2380 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2015 | East | 6 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2015 | West | 2003 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2015 | Total | 2009 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2014 | East | 3 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2014 | West | 2555 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2014 | Total | 2558 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2013 | East | 3 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2013 | West | 2643 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2013 | Total | 2646 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2012 | East | 16 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2012 | West | 2369 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2012 | Total | 2385 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2011 | East | 9 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2011 | West | 2819 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2011 | Total | 2828 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2010 | East | 10 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2010 | West | 2083 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2010 | Total | 2093 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2009 | East | No quota | |
| Greenland | Harbour porpoise | Phocoena phocoena | 2009 | West | 2029 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2009 | Total | 2029 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2008 | East | 1 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2008 | West | 1759 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2008 | Total | 1760 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2007 | East | 0 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2007 | West | 2912 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2007 | Total | 2912 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2006 | East | 1 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2006 | West | 2941 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2006 | Total | 2942 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2005 | East | 14 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2005 | West | 3200 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2005 | Total | 3214 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2004 | East | 18 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2004 | West | 2945 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2004 | Total | 2963 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2003 | East | 38 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2003 | West | 2287 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2003 | Total | 2325 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2002 | East | 2 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2002 | West | 2130 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2002 | Total | 2132 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2001 | East | 3 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2001 | West | 2213 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2001 | Total | 2216 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2000 | East | N/A | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2000 | West | 1605 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 2000 | Total | 1605 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 1999 | East | N/A | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 1999 | West | 1830 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 1999 | Total | 1830 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 1998 | East | N/A | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 1998 | West | 2131 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 1998 | Total | 2131 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 1997 | East | 1 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 1997 | West | 1592 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 1997 | Total | 1593 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 1996 | East | N/A | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 1996 | West | 1822 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 1996 | Total | 1822 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 1995 | East | N/A | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 1995 | West | 1427 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 1995 | Total | 1427 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 1994 | East | 71 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 1994 | West | 1663 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 1994 | Total | 1734 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 1993 | East | 83 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 1993 | West | 1638 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 1993 | Total | 1721 | No quota |
| Greenland | Harbour porpoise | Phocoena phocoena | 1992 | Total | *No reported catches | No quota |
This database of reported catches is searchable and information can be filtered by country, species or area. It is also possible to sort the information by the different columns, in ascending or descending order, by clicking the column you want to sort by and the associated arrows for the order. By default, 30 entries are shown, but this can be changed in the drop-down menu to show up to 100 entries per page.
Carry-over from previous years are included in the quota numbers, where this is applicable.
You can find the full catch database with all species here.
For any questions regarding the catch database, please contact the Secretariat at nammco-sec@nammco.org.
Other human impacts
By-catch
Harbour porpoises are subject to high levels of by-catch by commercial fisheries in most parts of their range, and in some areas it is considered to be a possible threat to porpoise populations.
The various types of commercial fishing gear harbour porpoises get caught in include: trawls, longlines, weirs and seines, but most are caught in pelagic or bottom set gill nets (Stenson 2003).
Monitoring and mitigation
In the late 1990s, concern over harbour porpoise by-catch levels led several organizations such as the International Whaling Commission (IWC), the International Council for the Exploration of the Sea (ICES) and NAMMCO to begin documenting and monitoring by-catch in the North Atlantic. At the same time, many governments began to institute management measures to reduce by-catch.
The North Sea
The North Sea is an area where high levels of by-catch occur. Between 1987-2001, some 5,700 porpoises were caught each year in the Danish North Sea bottom-set gillnet fisheries (Vinther and Larsen 2004). In the early 1990s, an estimated 2,200 harbour porpoises were by-caught each year in the English and Irish hake fisheries in the Celtic Sea (Hammond et al. 2013). Since that time, the EU has attempted to reduce by-catch with measures such as restricting Baltic Sea drift net fisheries, making acoustic deterrent devices or “pingers” mandatory in some gillnet fisheries in the North and Baltic Seas, and using onboard observers on larger vessels to monitor by-catch.
Norway
Northern Norway is another area where there are high levels of by-catch, with an estimated average of 2,900 porpoises caught annually between 2006 and 2014 (NAMMCO 2016), primarily in gillnet fisheries for cod and monkfish. The Norwegian Institute of Marine Research experimented with different types of pingers, or Acoustic Deterrent Devices, which resulted in a 70-100% reduction of bycatch of harbour porpoises. The Norwegian Ministry of Trade, Industry and Fisheries has made the use of pingers mandatory during the cod fishery in Vestfjorden (which is responsible for rougly a third of all porpoise bycatch in Norway) from 2021 (National Progress Report Norway 2020).
Iceland
Around Iceland, by-catch has been reported to be around 200 animals per year (NAMMCO 2015). This is based solely on reports by fishermen, so is likely a minimum number. Harbour porpoises were previously by-caught in salmon drift gillnet fisheries on the west coast of Greenland, but this fishery closed in 1976 (Stenson 2003).
The Northwest Atlantic
In the Northwest Atlantic, high rates of by-catch in the U.S. and Canadian ranges of harbour porpoise during the 1990s led to the implementation of several management initiatives (primarily in the U.S.) to reduce by-catch to sustainable levels. These measures have been successful to the point where current levels of by-catch are not considered to pose a threat to harbour porpoises in this region (COSEWIC 2006). There is less information available for waters further north, in the Gulf of St. Lawrence and around Newfoundland and Labrador. By-catch was estimated to be several thousand per year during the early 1990s (Stenson 2003). Reduction in fishing effort, particularly the use of gill nets, has likely led to a reduction in by-catch since that time, but this has not been well monitored.
Noise/disturbance
Due to its near shore distribution, harbour porpoises are exposed to various types of human activity throughout their range. Noise is a particular concern since porpoises use sound extensively in their foraging activities. It has been shown that porpoises feed almost constantly on around three thousand small fish per day (Wisniewska et al. 2016, Wisniewska et al. 2018). This behaviour makes these animals vulnerable to any disturbance that may affect their foraging and thereby their long-term fitness.
Types of underwater noise & impacts
Underwater noise may be divided into ambient background noise and man-made anthropogenic noise. The first is caused by natural sources like waves, wind, earth quakes and animals, while the other is caused by sources such as ships, military operations, oil and gas exploitation and extraction, wind turbines, bridges and building of offshore constructions.
The effect of noise on marine mammals can be determined on several levels: Effects on individuals (from changes in behaviour to survival); Changes in relative densities on a local scale; Changes in distribution and abundance; Population level effects.
Wind farms
Construction of wind farms in the North Sea, which involves driving piles into the sea floor, is a source of significant noise. Harbour porpoises have been observed to move away from sites where pile driving is occurring, generally to about a 20 km distance (Kastelein et al. 2018), which may result in loss of foraging opportunities. In some cases, noise reducing measures, such as “bubble curtains” have been used at these construction sites, which appear to greatly reduce the area of disturbance for harbour porpoises (Peschko et al. 2016).
Studies looking at the effects from operating wind farms have shown mixed results. Negative long-term effects have been documented in the Baltic Sea (Teilmann and Carstensen 2012). No effects were seen in the eastern North Sea (Tougaard et al. 2006), while positive effects were observed in the southern North Sea (Scheidat et al. 2011). These diverging results may be related to factors such as: i) an increase in noise level due to turbine noise, ii) high motivation to follow fish schools leading to ignoring any noise increase, or iii) an increase in fish abundance due to reduced fishing activities. This means that similar offshore constructions may have very different effects on harbour porpoises depending on the context.
Although offshore constructions may create noise, they may also create artificial reefs with increased productivity and biodiversity that can also actually benefit top predators like the harbour porpoise.
Vessel traffic
Another potential source of noise and disturbance is vessel traffic. Analysis of harbour porpoises tagged in the Danish Belt Seas has shown that these animals experience ship noise from 17-89% of the time (Wisniewska et al. 2018). The study also showed a significant reduction in foraging echolocation (buzzing) at levels of received ship noise that were not unusual in Danish waters.
Seismic airguns
In a recent study using play-back signals from a single 10 inch3 airgun every 2-3 sec for 1 minute results showed that some animals responded with changes in horizontal movements (faster, longer and more directed) and shorter and shallower dives for up to 8 hours, with an additional 24 hours taken to resume baseline behaviour, while others did not show a response (van Beest et al. 2018). This indicates that the response of the animals to similar stimuli may depend on individual robustness to disturbances.
Contaminants
Like other marine mammals, harbour porpoises ingest persistent organic pollutants (POPs) in their diet, as they consume fish which have been exposed to these compounds from coastal runoff and human industrial activities. POPs are lipophilic compounds and as such will accumulate in the lipid-rich blubber of porpoises, leading to higher levels in the tissues as the porpoises get older. In females, however, some of their contaminant burden is passed on to the calf during gestation and lactation, which may give the calf a higher level of POPs than its mother (Imazaki et al. 2015).
Contaminant levels
Studies on harbour porpoises have shown that the animals in the North Sea, inner Danish waters and the Baltic Sea have accumulated high levels of a wide range of organochlorine contaminants such as polychlorinated biphenyls (PCBs), dioxins, hexachlorocyclohexanes (HCHs) and DDT/DDE (Strand et al. 2005).
Contaminant levels vary by region: porpoises stranded on the Belgian North Sea coast had relatively low amounts of organochlorine pesticides (DDTs, hexachlorobenzene (HCB), and HCHs) in their tissues, but higher levels of PCBs (Covaci et al. 2002). High levels of butyltin and mercury, within the range of 68-4,605 mg BT/kg and 0.22-92 mg Hg/kg, were found in the liver of harbour porpoises stranded or by-caught in Danish waters, while lower levels were seen in porpoises from West Greenland (Strand et al. 2005).
Effects of contaminants
The effects of the presence of these contaminants is hard to determine, especially since there may be synergistic effects occurring when more than one type of pollutant is present (Imazaki et al. 2015). One potential effect is suppression of the immune system, which could lead to increased mortality from infectious diseases. Another is that PCBs, DDT, and DDE may interfere with thyroid function in porpoises, leading to severe interfollicular fibrosis (Das et al. 2006 in Imazaki et al. 2015). Another concern is that some POPs disrupt the endocrine and reproductive systems of marine mammals, which could lead to reproductive difficulties or failure.
Climate change
Climate change could have a number of impacts on harbour porpoises either directly or indirectly. For example, if water temperatures increase, this could have an impact on the habitat available for harbour porpoises and consequently their distribution. Harbour porpoises are currently residing longer in West Greenland than previously seen, perhaps due to warming seas there. Changes in water temperature could also affect the distribution of the porpoises’ prey species.
Change in diet and increase in parasites
In Greenland, an increase in the severity of parasitic infections and the emergence of new parasite species has been observed compared to a previous study of porpoises from 1995 (Lehnert et al. 2014). This is probably occurring due to changes in the porpoises’ diet from increasing sea temperatures and receding ice cover. Another study in Greenland found that porpoises sampled in 2009 had a more varied diet than those sampled in 1995, with 23 different major prey items found in the recent stomachs compared to 11 previously (Heide-Jørgensen et al. 2011). New items were also found in the 2009 samples that were not present before; Atlantic cod (Gadus morhua) and Greenland cod (G. ogaq). The presence of these fish is thought to be due to the warmer waters occurring off West Greenland as a result of climate change.
Ice entrapments
Another impact of climate change has been observed in Norway, where in some fjords, porpoises become trapped when freshwater input from a river freezes and forms a layer of ice on top of the seawater (Bjørge and Tolley 2018). With warmer air temperatures, there is increased runoff from melting glaciers and so increased freshwater input into these fjords, with the result that these entrapments may become increasingly common.
Cumulative Impact
The 2019 meeting of the NAMMCO Harbour Porpoise Working Group, which produced an assessment of the population in West Greenland, noted that specific and systematic monitoring of all the different non-hunting related anthropogenic stressors on harbour porpoises and their potential to have cumulative impacts is not currently being systematically performed. It suggested that further research on life history parameters and dietary shifts from existing samples could be one relevant approach to investigate these impacts further. It was also noted that although these stressors may be having an impact on an individual level, they did not as yet appear to be having population level impacts in Greenland.
Research in NAMMCO member countries
Research into the abundance, life history, ecology and population dynamics of harbour porpoises is ongoing in several NAMMCO member countries. All the NAMMCO countries were, for example, involved in the Joint IMR/NAMMCO International Workshop on the Status of Harbour Porpoises in the North Atlantic (conducted 2018, published 2019), the report of which provides a comprehensive review of available information and knowledge gaps.
Greenland
In Greenland, two harbour porpoises were live captured and outfitted with satellite tags to track their movements. The success of that study has led Pinngortitaleriffik/the Greenland Institute of Natural Resources to initiate a larger study to investigate the porpoises’ migration behaviour, habitat use around Greenland, and their role in the west Greenland marine food chain. 30 harbour porpoises were thus caught and instrumented with satellite transmitters. These porpoises displayed long-range oceanic movements, in contrast to 71 porpoises tagged in Danish waters which did not leave the continental shelf (Nielsen et. al 2018). Greenland also collects tissue samples from harvested porpoises for various analyses and monitors harvest numbers.
Norway
Significant research in Norway has been dedicated to methods for mitigating porpoise by-catch in commercial fisheries, especially in Northern Norway. The Norwegian Institute for Marine Research (IMR) is currently running experiments with Acoustic Deterrent Devices (ADDs), called pingers, on large-mesh gillnets in the coastal zone. Thus far, the use of pingers has resulted in a 70-100% reduction of porpoise bycatch when compared to nets without pingers (National Progress Report Norway 2018). Since 2021, the use of pingers is mandatory during the cod fishery in Vestfjorden (National Progress Report Norway 2020).
By-caught harbour porpoises are also collected by the IMR for biological sampling, and a food-web model is being developed for the Vestfjord area near Lofoten to study the role of harbour porpoises in this region (NAMMCO 2017).
Researchers at the IMR are also carrying out studies on harbour porpoise ecology and population biology using by-caught animals from 2016, 2017, 2018 and 2019.
Research on pollutants and population structure have also been recently published. In the work on pollutants, the concentrations of phthalate metabolites were found to be higher in animals inhabiting waters adjacent to areas with higher human activity and populations (Rian et al. 2020). The amount of phthalic acid was negatively associated with the body size, while gender differences were not identified. The study indicated that the harbour porpoise can be potentially used as tracers of phthalate (plasticizers) pollution in the marine environment. In the study on population structure, Quintela et al. (2020) concluded that the 134 individuals analysed did not reveal any spatial structure, i.e. the existence of only one population of harbour porpoise in Norwegian coastal waters. However, a more extensive sampling, both in terms of numbers of individuals and in geographic scope, preferably covering both sides of the Atlantic, was highlighted as essential to elucidate the genetic structure of harbour porpoises.
Iceland
Iceland also conducts research into levels of harbour porpoise by-catch in commercial fisheries and methods of mitigating this. Pingers were tested for the first time in the Icelandic cod gillnet fishery in April 2017, but their use showed no reduction in porpoise by-catch. A different type of porpoise deterrent, the Porpoise Alerting Device (PAL) was tested in 2018, with the same results. A pinger with a different signal was tested in 2019 and no porpoises were caught. Further trials with this pinger will be conducted in April 2020. (National Progress Report Iceland 2018, 2019).
Genetic studies are ongoing in Iceland as well, in a collaboration between the MFRI and the University of Potsdam. Among the objectives of this study is estimation of population size based on close kin analysis. Over 2,100 Icelandic harbour porpoises have now been genotyped at 13 microsatellite loci (National Progress Report Iceland 2019). This data will be analysed regarding affinity of Icelandic porpoises to other regions of the North Atlantic as well as with regard to population structure within Iceland. Since 2017, fishermen received a payment for each harbour porpoise DNA tissue sample that they sent in to the MFRI, and this resulted in an increased number of samples obtained. In 2019 received samples were 135, which is less than the around 200 samples that were received in the earlier years (National Progress Report Iceland 2019). Although no payment will be offered to fishermen in 2020, samples will continue to be included from by-caught porpoises in the annual gillnet survey around Iceland in the spring (18 in 2019) as well as samples from any stranded animals.
A recent genetic study, which included samples from Iceland, has also developed single nucleotide polymorphisms (SNPs) for porpoises. New analyses on these SNPs have been performed on 150 harbour porpoise specimens from the North Atlantic, including 12 specimens from Iceland. These analyses yielded 26,320 informative SNPs, which are currently being used for population structure assessment across the entire North Atlantic (National Progress Report Iceland 2019).
In 2020, the University of Iceland (UI) and the Marine and Freshwater Research Institute (MFRI) have analysed samples of stranded or bycaught harbour porpoises from the MFRI tissue bank to investigate their trophic ecology (National Progress Report Iceland 2020). This was done as part of a larger study that investigates the diet of killer whales.
Continuous porpoise detectors (C-PODS) have also been deployed annually since 2018 in Skjálfandi Bay for detection of harbour porpoises (National Progress Report Iceland 2018, 2019, 2020).
Aarefjord, H., Bjørge, A., Kinze, C.C. and Lindstedt, I. (1995). Diet of the harbour porpoise (Phocoena phocoena) in Scandinavian waters. Reports of the International Whaling Commission, Special Issue 16, 211-222.
Alfonsi, E, Hassani, S, Carpentier, F.-G., Le Clec’h, J.-Y., Dabin, W., Van Canneyt, O., Fontaine, M.C. and Jung, J.-L. (2012). A European Melting Pot of Harbour Porpoise in the French Atlantic Coasts Inferred from Mitochondrial and Nuclear Data. PLoS ONE, 7(9), 1-11. https://doi.org/10.1371/journal.pone.0044425
Andersen, L.W. (2003). Harbour porpoises (Phocoena phocoena) in the North Atlantic: Distribution and genetic population structure. NAMMCO Scientific Pubmmlications, 5, 11-30. https://doi.org/10.7557/3.2737
Andersen, L.W., Holm, L.-E., Siegismund, H.R., Clausen, B., Kinz, C.C. and Loeschcke, V. (1997). A combined DNA-microsatellite and isozyme analysis of the population structure of the harbour porpoise in Danish waters and West Greenland. Heredity, 78(3), 270-276. https://doi.org/10.1038/hdy.1997.41
Andreasen, H., Ross, S.D., Siebert, U., Andersen, N.G., Ronnenberg, K. and Gilles, A. (2017). Diet composition and food consumption rate of harbor porpoises (Phocoena phocoena) in the western Baltic Sea. Marine Mammal Science, 33(4), 1053-1079. https://doi.org/10.1111/mms.12421
Arnold, P.W. (1972). Predation on harbour porpoise, Phocoena phocoena, by a white shark, Carcharodon carcharias. Canadian Journal of Fisheries and Aquatic Sciences, 29, 1213-1214. https://doi.org/10.1139/f72-179
Bjørge, A. and Tolley, K.A. (2018). Harbor porpoise (Phocoena phocoena). In B. Wursig, J.G.M. Thewissen and K.M. Kovacs (eds.), Encyclopedia of Marine Mammals (3rd ed., pp. 448-451). Academic Press. https://doi.org/10.1016/B978-0-12-804327-1.00144-8
Bjørge, A. and Øien, N. (1995). Distribution and abundance of harbour porpoise, Phocoenea phocoena, in Norwegian waters. Reports of the International Whaling Commission, Special Issue 16, 89-98.
Bouveroux, T., Kiszka, J.J., Heithaus, M.R., Jauniaux, T. and Pezeril, S. (2014). Direct evidence for gray seal (Halichoerus grypus) predation and scavenging on harbor porpoises (Phocoena phocoena). Marine Mammal Science 30(4), 1542-1548. https://doi.org/10.1111/mms.12111
Camphuysen, K.C.J. (2004). The return of the harbour porpoise (Phocoena phocoena) in Dutch coastal waters. Lutra, 47 (2), 113-122. https://zoogdierwinkel.nl/content/lutra-47-2-2004
Carlström, J. (2005). Diel variation in echolocation behaviour of wild harbour porpoises. Marine Mammal Science, 21(1), 1-12. https://doi.org/10.1111/j.1748-7692.2005.tb01204.x
Clausen, K.T., Wahlberg, M., Beedholm, K., Deruiter, S. and Teglberg Madsen, P. (2011). Click communication in harbour porpoises Phocoena phocoena. Bioacoustics, 20(1), 1-28. https://doi.org/10.1080/09524622.2011.9753630
Committee on the Status of Endangered Wildlife in Canada (COSEWIC). (2006). COSEWIC assessment and update status report on the harbour porpoise Phocoena phocoena (Northwest Atlantic population) in Canada. vii + 32 pp.
Covaci, A., Van De Vijver, K., DeCoen, W., Dasc, K., Bouquegneau, J.M., Blust, R. and Schepens, P.(2002). Determination of organohalogenated contaminants in liver of harbour porpoises (Phocoena phocoena) stranded on the Belgian North Sea coast. Marine Pollution Bulletin, 44(10), 1157–1165. https://doi.org/10.1016/S0025-326X(02)00147-9
Cucknell, A.-C., Boisseau, O., Leaper ,R. and McLanaghan, R. (2017) Harbour porpoise (Phocoena phocoena) presence, abundance and distribution over the Dogger Bank, North Sea, in winter. Journal of the Marine Biological Association of the United Kingdom, 97(7), 1455-1465. https://doi.org/10.1017/S0025315416000783
Culik, B., von Dorrien, C., Müller, V. and Conrad, M. (2015). Synthetic communication signals influence wild harbour porpoise (Phocoena phocoena) behaviour. Bioacoustics, 24(3), 201-221. http://dx.doi.org/10.1080/09524622.2015.102384
Donovan, G.P. and Bjørge, A. (1995). Harbour porpoises in the North Atlantic: edited extract from the Report of the IWC Scientific Committee, Dublin 1995. Reports of the International Whaling Commission, 16, 3-25.
Fontaine, P., Hammill, M.O., Barrette, C. and Kingsley, M.C. (1994). Summer diet of the harbour porpoise (Phocoena phocoena) in the estuary and the northern Gulf of St. Lawrence. Canadian Journal of Fisheries and Aquatic Sciences, 51, 172-178. https://doi.org/10.1139/f94-019
Gaskin, D.E. (1992). Status of the harbour porpoise, Phocoena phocoena, in Canada. Canadian Field-Naturalist, 106, 36-54.
Gilles, A., Gunnlaugsson, Th., Mikkelsen, B., Pike, D.G. and Víkingsson, G. (2011). Harbour porpoise Phocoena phocoena summer abundance in Icelandic and Faroese waters, based on aerial surveys in 2007 and 2010. NAMMCO/SC/18/AESP/11.
Haelters, J., Kerckhof, F., Jacques, T.G. and Degraer, S. (2011). The harbour porpoise Phocoena phocoena in the Belgian part of the North Sea: trends in abundance and distribution. Belgian Journal of Zoology, 141(2), 75-84. http://files.belgianjournalofzoology.eu/download/BJZ_141_2_colour.pdf
Hammond, P.S., Lacey, C., Gilles, A. … Øien, N. (2017). Estimates of cetacean abundance in European Atlantic waters in summer 2016 from the SCANS-III aerial and shipboard surveys. SCANS III final report. pp. 40.
Hammond, P.S., Macleod, K., Berggren, P. … Vazquez, J.A. (2013). Cetacean abundance and distribution in European Atlantic shelf waters to inform conservation and management. Biological Conservation, 164, 107-122. https://doi.org/10.1016/j.biocon.2013.04.010
Hammond, P.S., Bearzi, G., Bjørge, A., Forney, K., Karczmarski, L., Kasuya, T., Perrin, W.F., Scott, M.D., Wang, J.Y., Wells, R.S. and Wilson. (2008). Phocoena phocoena. The IUCN Red List of Threatened Species 2008: e.T17027A6734992. http://dx.doi.org/10.2305/IUCN.UK.2008.RLTS.T17027A6734992.en.
Hammond, P.S., Berggren, P., Benke, Borchers, H. D.L., Collet, A., Heide‐Jørgensen M.P., Heimlich, S., Hiby, A.R., Leopold M.F. and Øien, N. (2002). Abundance of harbour porpoise and other cetaceans in the North Sea and adjacent waters. Journal of Applied Ecology, 39, 361-376. https://doi.org/10.1046/j.1365-2664.2002.00713.x
Hasselmeier, I., Abt, K.F., Adelung, D.,Siebert, U. (2004). Stranding patterns of Harbour Porpoises (Phocoena phocoena) in the North and Baltic Seas: when does the birth period occur? Journal of Cetacean Research and Management, 6(3), 59-263. https://archive.iwc.int/pages/search.php?search=%21collection15&k=
Heide-Jørgensen, M.P. (2013). Correction of at-surface abundance of harbour porpoises in West Greenland based on detection to 1 m depth. NAMMCO SC/20/HP/11 Harbour porpoise Working Group November 2013, 1.
Heide‐Jørgensen, M.P., Iversen, M., Nielsen, N.H., Lockyer, C., Stern, H., Ribergaard, M.H. (2011). Harbour porpoises respond to climate change. Ecology and Evolution, 1(4), 579-585. https://doi.org/10.1002/ece3.51
Herreras, M.V., Kaarstad, S.E., Balbuena, J.A., Kinze, C.C. and Raga, J.A. (1997). Helminth parasites of the digestive tract of the harbour porpoise (Phocoena phocoena) in Danish waters: a comparative geographical analysis. Diseases of Aquatic Organisms, 28(3), 163-167. https://doi.org/10.3354/dao028163
IJsseldijk, L.L., Camphuysen, K.C.J., Nauw, J.J. and Aarts, G. (2015). Going with the flow: Tidal influence on the occurrence of the harbour porpoise (Phocoena phocoena) in the Marsdiep area, The Netherlands. Journal of Sea Research, 103, 129-137. https://doi.org/10.1016/j.seares.2015.07.010
Imazaki, P.H., Brose, F., Jauniaux, T., Das, K. and Muller, M. (2015). Estrogenic evaluation and organochlorine identification in blubber of North Sea harbour porpoise (Phocoena phocoena) stranded on the North Sea coast. BioMed Research International, 2015, 1-13. https://doi.org/10.1155/2015/438295
Johnston, D.W., Westgate, A.J. and Read, A.J. (2005). Effects of fine-scale oceanographic features on the distribution and movements of harbour porpoises Phocoena phocoena in the Bay of Fundy. Marine Ecology Progress Series, 295, 279-293. https://doi.org/10.3354/meps295279
Kastelein, R.A., Helder-Hoek, L. and Jennings, N. (2018). Seasonal changes in food consumption, respiration rate, and body condition of a male harbor porpoise (Phocoena phocoena). Aquatic Mammals, 44(1), 76-91. https://doi.org/10.1578/AM.44.1.2018.76
Kesselring, T., Viquerat, S., Brehm, R. and Siebert, U. (2017). Coming of age: – Do female harbour porpoises (Phocoena phocoena) from the North Sea and Baltic Sea have sufficient time to reproduce in a human influenced environment? PLoS ONE, 12(10): 1-14. https://doi.org/10.1371/journal.pone.0186951
Kyhn, L.A., Tougaard, J., Beedholm, K., Jensen, F.H., Ashe, E., Williams, R. and Madsen, P.T. (2013). Clicking in a killer whale habitat: narrow-band, high-frequency biosonar clicks of harbour porpoise (Phocoena phocoena) and Dall’s porpoise (Phocoenoides dalli). PLoS ONE, 8(5), 1-12. https://doi.org/10.1371/journal.pone.0063763
Lawson, J.W. and Gosselin, J.-F. (2009). Distribution and preliminary abundance estimates for cetaceans seen during Canada’s Marine Megafauna Survey – A component of the 2007 TNASS. Canadian Scientific Advisory Secretariat. Res. Doc. 208/031. 33 pp. http://www.dfo-mpo.gc.ca/csas-sccs/publications/resdocs-docrech/2009/2009_031-eng.htm
Learmonth, J.A., Murphy, S., Luque, P.L., Reid, R.J., Patterson, I.A.P., Brownlow, A., Ross, H.M., Barley, J.P., Santos, M.B. and Pierce, G.J. (2014) Life history of harbor porpoises (Phocoena phocoena) in Scottish (UK) waters. Marine Mammal Sciences, 30, 1427-1455. https://doi.org/10.1111/mms.12130
Lehnert, K., Seibel, H., Hasselmeier, I., Wohlsein, P., Iversen, M., Nielsen, N.H., Heide-Jørgensen, M.P., Prenger-Berninghoff, E. and Siebert, E. (2014). Increase in parasite burden and associated pathology in harbour porpoises (Phocoena phocoena) in West Greenland Polar Biology, 37(3), 321-331. https://doi.org/10.1007/s00300-013-1433-2
Linnenschmidt, M., Teilmann, J., Akamatsu, T., Dietz, R. and Miller, L.A. (2013). Biosonar, dive, and foraging activity of satellite tracked harbor porpoises (Phocoena phocoena). Marine Mammal Science, 29(2), E77–E97. https://doi.org/10.1111/j.1748-7692.2012.00592.x
Lockyer, C. (2003). Harbour porpoises (Phocoena phocoena) in the North Atlantic: Biological parameters. NAMMCO Scientific Publications, 5, 71-89. https://doi.org/10.7557/3.2740
North Atlantic Marine Mammal Commission (NAMMCO). (2019). Report of the Abundance Estimates Working Group, October 2019, Tromsø, Norway. https://nammco.no/topics/abundance_estimates_reports/
North Atlantic Marine Mammal Commission (NAMMCO) and the Norwegian Institute of Marine Research (IMR). (2019). Report of Joint IMR/NAMMCO International Workshop on the Status of Harbour Porpoises in the North Atlantic, December 2018, Tromsø, Norway.
North Atlantic Marine Mammal Commission (NAMMCO). (2018). Report of the workshop “Cetacean abundance and distribution in the North Atlantic”, October 2017, Halifax, Canada. https://nammco.no/topics/abundance_estimates_reports/
North Atlantic Marine Mammal Commission (NAMMCO). (2017). Report of the 24th Scientific Committee meeting, Reykjavík, Iceland (14-17 November). https://nammco.no/topics/scientific-committee-reports/
North Atlantic Marine Mammal Commission (NAMMCO). (2016). NAMMCO Annual Report 2016. NAMMCO, Tromsø, Norway, pp. 363. https://nammco.no/topics/annual-reports/
North Atlantic Marine Mammal Commission (NAMMCO). (2015). NAMMCO Annual Report 2015. NAMMCO, Tromsø, Norway, pp. 374. https://nammco.no/topics/annual-reports/
Nielsen, N.H. and Heide-Jørgensen, M.P. (2013). Catch statistics for harbour porpoises in West Greenland including correction for unreported catches. NAMMCO SC/20/HP/06 Harbour Porpoise working group November 2013, 1-17. https://nammco.no/wp-content/uploads/2019/02/sc20-hp-06-historic-catch-statistics-of-harbour-porpoises_new.pdf
Nielsen, N.H., Hansen, R.G., Teilmann, J. and Heide-Jørgensen, M.P. (2013). Extensive offshore movements of harbour porpoises (Phocoena phocoena). NAMMCO SC/20/HP/08 Harbour porpoise working group November 2013, 1-12. https://nammco.no/wp-content/uploads/2019/02/sc20-hp-08-nielsen-et-al-offshore-migrations-of-harbour-porpoises.pdf
Nielsen, N.H., Teilmann, J., Sveegaard, S., Hansen, R.G., Sinding, M.-H. S., Dietz, R. and Heide-Jørgensen, M.P. (2018). Oceanic movements, site fidelity and deep diving in harbour porpoises from Greenland show limited similarities to animals from the North Sea. Marine Ecology Progress Series, 597, 259–272. https://doi.org/10.3354/meps12588
National Oceanic and Atmospheric Administration (NOAA). (2016). Harbor Porpoise (Phocoena phocoena phocoena): Gulf of Maine/Bay of Fundy Stock. May 2016. pp. 13.
Osinga, N., Hart, P. and Morick, D. (2008). By-catch and drowning in harbour porpoises (Phocoena phocoena) stranded on the northern Dutch coast. European Journal of Wildlife Research, 54, 667-674. https://doi.org/10.1007/s10344-008-0193-8
Palme, A., Laikre, L., Utter, F. and Ryman, N. (2008). Conservation genetics without knowing what to conserve: the case of the Baltic harbour porpoise Phocoena phocoena. Oryx, 42(2), 305-308. https://doi.org/10.1017/S0030605308006960
Peschko, V., Ronnenberg, K., Siebert, U. and Gilles, A. (2016). Trends of harbour porpoise (Phocoena phocoena) density in the southern North Sea. Ecological Indicators, 60, 174-183. https://doi.org/10.1016/j.ecolind.2015.06.030
Pierpoint, C. (2008). Harbour porpoise (Phocoena phocoena) foraging strategy at a high energy, near-shore site in south-west Wales, UK. Journal of the Marine Biological Association of the United Kingdom, 88(6), 1167-1173. https://doi.org/10.1017/S0025315408000507
Pike, D.G., Paxton, C.G.M., Gunnlaugsson, T. and Víkingsson, G. (2009). Trends in distribution and abundance of cetaceans from aerial surveys in Icelandic coastal waters, 1986–2001. NAMMCO Scientific Publications, 7, 117-142. https://doi.org/10.7557/3.2710
Polacheck, T., Wenzel, F.W. and Early, G. (1995). What do stranding data say about harbor porpoises (Phocoena phocoena)? Reports of the International Whaling Commission, Special Issue 16, 169-179.
Quintela, M., Besnier, F., Bjørghild S., Glover, K.A. and Lindstrøm, U. (2020). Population structure of bycaught harbour porpoise (Phocoena phocoena) in Norway. Marine Biology Research, 16(3), 141-147. https://doi.org/10.1080/17451000.2020.1729992
Read, A.J. and Hohn, A.A. (1995). Life in the fast lane: the life history of harbour porpoises from the Gulf of Maine. Marine Mammal Science, 11, 423-440. https://doi.org/10.1111/j.1748-7692.1995.tb00667.x
Read, A.J. and Westgate, A.J. (1997). Monitoring the movements of harbour porpoises (Phocoena phocoena) with satellite telemetry. Marine Biology, 130, 315-322. https://doi.org/10.1007/s002270050251
Rian, M.B., Vike-Jonas, K., Gonzalez, S.V., Ciesielski, T.M., Venkatraman, V., Lindstrøm, U., Jenssen, B.M. and Asimakopoulos, A.G. (2020). Phthalate metabolites in harbor porpoises (Phocoena phocoena) from Norwegian coastal waters. Environment International, 137, 105525. https://doi.org/10.1016/j.envint.2020.105525
Ross, H.M. and Wilson, B. (1996). Violent interactions between bottlenose dolphins and harbour porpoises. Proceedings of the Royal Society B, 263(1368), 283-286. https://doi.org/10.1098/rspb.1996.0043
Santos, M.B. and Pierce, G.J. (2003). The diet of harbour porpoise (Phocoena phocoena) in the northeast Atlantic. Oceanography and Marine Biology, 41, 355-390. https://www.researchgate.net/publication/228580356_The_diet_of_harbour_porpoise_Phocoena_phocoena_in_the_northeast_Atlantic
Santos, M.B., Pierce, G.J., Learmonth, J.A., Reid, R.J., Ross, H.M., Patterson, I.AP., Reid, D.G. and Beare, D. (2004). Variability in the diet of harbor porpoises (Phocoena phocoena) in Scottish waters 1992-2003. Marine Mammal Science, 20(1), 1-27. https://doi.org/10.1111/j.1748-7692.2004.tb01138.x
Scheidat, M., Tougaard, J., Brasseur, S., Carstensen, J., von Polanen Petel, T., Teilmann, J. and Reijnders, P. (2011). Harbour porpoises (Phocoena phocoena) and wind farms: a case study in the Dutch North Sea. Environmental Research Letters, 6(2), 025102. https://doi.org/10.1088/1748-9326/6/2/025102
Sharpe, M. & Berggren, P. 2023. Phocoena phocoena (Europe assessment). The IUCN Red List of Threatened Species 2023: e.T17027A219010660. Accessed on 21 December 2023.
Siebert, U., Gilles, A., Lucke, K., Ludwig, M., Benke, H., Kock, K.-H. and Scheidata, M. (2006). A decade of harbour porpoise occurrence in German waters – analyses of aerial surveys, incidental sightings and strandings. Journal of Sea Research, 56, 65-80. https://doi.org/10.1016/j.seares.2006.01.003
Sørensen, T.B. and Kinze, C. (1994). Reproduction and reproductive seasonality in Danish harbour porpoises (Phocoena phocoena). Ophelia, 39(3), 159-176. https://doi.org/10.1080/00785326.1994.10429541
Spitz J., Rousseau, Y. and Ridoux, V. (2006). Diet overlap between harbour porpoise and bottlenose dolphin: An argument in favour of interference competition for food? Estuarine Coastal and Shelf Science, 70(1-2), 259-270. https://doi.org/10.1016/j.ecss.2006.04.020
Stenson, G.B. (2003). Harbour porpoise (Phocoena phocoena) in the North Atlantic: Abundance, removals, and sustainability of removals. NAMMCO Scientific Publications, 5, 271-302. https://doi.org/10.7557/3.2830
Strand, J., Larsen, M.M. and Lockyer, C. (2005). Accumulation of organotin compounds and mercury in harbour porpoises (Phocoena phocoena) from the Danish waters and West Greenland. Science of the Total Environment, 350(1-3), 59-71. https://doi.org/10.1016/j.scitotenv.2005.02.038
Stringell, T., Hill, D., Rees, D. et al. (2015). Predation of harbour porpoises (Phocoena phocoena) by grey seals (Halichoerus grypus) in Wales. Aquatic Mammals, 41(2), 188-191. https://doi.org/10.1578/AM.41.2.2015.188
Sveegaard S, Teilmann J, Tougaard J., Dietz, R., Mouritsen, K.N., Desportes, G. and Siebert, U. (2011). High-density areas for harbor porpoises (Phocoena phocoena) identified by satellite tracking. Marine Mammal Science, 27(1), 230-246. https://doi.org/10.1111/j.1748-7692.2010.00379.x
Teilmann, J. and Carstensen, J. (2012). Negative long term effects on harbour porpoises from a large scale offshore wind farm in the Baltic—evidence of slow recovery. Environmental Research Letters, 7(4), 045101. https://doi.org/10.1088/1748-9326/7/4/045101
Todd, V.L.G., Pearse, W.D., Tregenza, N.C., Lepper, P.A. and Todd, I.B. (2009). Diel echolocation activity of harbour porpoises (Phocoena phocoena) around North Sea offshore gas installations. ICES Journal of Marine Science, 66, 734-745. https://doi.org/10.1093/icesjms/fsp035
Tougaard, J., Carstensen, J., Wisz, M.S., Jespersen, M., Teilmann, J., Bech, N.I. and Skov, H. (2006). Harbour Porpoises on Horns Reef – Effects of the Horns Reef Wind Farm (Final Report to Vattenfall A/S). https://porpoise.org/library/harbour-porpoises-horns-reef-effects-horns-reef-wind-farm-final-report/
van Beest, F.M., Teilmann, J., Hermannsen, L., Galatius, A., Mikkelsen, L., Sveegaard, S., Balle, J., Dietz, R. and Nabe-Nielsen, J. (2018). Fine-scale movement responses of free-ranging harbour porpoises to capture, tagging and short-term noise pulses from a single airgun. Royal Society Open Science, 5(1), 170110. https://doi.org/10.1098/rsos.170110
Verfuß, U.K., Honnef, C.G. and Benke, H. (2006). Seasonal and geographical variation of harbour porpoise (Phocoena phocoena) habitat use in the German Baltic Sea monitored by passive acoustic methods (PODs). In von Nordheim H, Boedeker, D. and Krause, J.C. (eds.), Progress in Marine Conservation in Europe (pp. 209-224). Berlin, Heidelberg: Springer. https://doi.org/10.1007/3-540-33291-X_13
Víkingsson, G.A., Ólafsdóttir, D. and Sigurjónsson, J. (2003). Diet of harbour porpoises (Phocoenea phocoena) in Icelandic coastal waters. NAMMCO Scientific Publications, 5, 243-270. https://doi.org/10.7557/3.2829
Vinther, M. and Larsen, F. (2004). Updated estimates of harbour porpoise (Phocoena phocoena) bycatch in the Danish North Sea bottom-set gillnet fishery. Journal of Cetacean Research and Management, 6(1), 19-24. https://archive.iwc.int/pages/search.php?search=%21collection15&k=
Waring, G.T., Josephson, E., Maze-Foley, K. and Rosel, P. (eds.). (2016). US Atlantic and Gulf of Mexico Marine Mammal Stock Assessments – 2015. NOAA Tech Memo NMFS NE 238. pp. 501. https://doi.org/10.7289/V57S7KTN
Wisniewska, D.M., Johnson, M., Teilmann, J., Rojano-Doñate, L., Shearer, J., Sveegaard, S., Miller, L.A., Siebert, U. and Madsen, P.T. (2016). Ultra-High Foraging Rates of Harbor Porpoises Make Them Vulnerable to Anthropogenic Disturbance. Current Biology, 26(11), 1441–1446. https://doi.org/10.1016/j.cub.2016.03.069
Wisniewska, D.M., Johnson, M., Teilmann, J., Rojano-Doñate, L., Shearer, J., Sveegaard, S., Miller, L.A., Siebert, U. and Madsen, P.T. (2018). Response to “Resilience of harbor porpoises to anthropogenic disturbance: Must they really feed continuously?” Marine Mammal Science, 34(1), 265–270. https://doi.org/10.1111/mms.12463
Wright, A.J., Maar, M., Mohn, C., Nabe-Nielsen, J., Siebert, U., Jensen, L.F., Baagøe, H.J. and Teilmann, J. (2013). Possible causes of a harbour porpoise mass stranding in Danish waters in 2005. PLoS ONE, 8(2), 1-14. https://doi.org/10.1371/journal.pone.0055553