Atlantic White-Sided Dolphin
Updated: June 2020
The Atlantic white-sided dolphin (Lagenorhynchus acutus) is a large dolphin named for the distinctive light-coloured patches along its sides. It is more colourful than other species of dolphins, with light and dark grey, black, yellow and white colouration. Their beak is short and not distinctly separated from the melon. They have long, pointed flippers, and a dorsal fin that is relatively tall and strongly curved. The Atlantic white-sided dolphin is found in the temperate and sub-polar waters of the North Atlantic, mainly in continental shelf waters. They appear to prefer areas where there are steep slopes and, in some cases, underwater canyons. The Atlantic white-sided dolphin may easily be misidentified as the white-beaked dolphin (Lagenorhynchus albirostris), as their ranges overlap considerably. The white-sided dolphin is typically smaller than the white-beaked, and it has the distinctive white and yellow streaks on its side.
ABUNDANCE
Atlantic white-sided dolphins are abundant throughout their range, although there are few surveys available to provide population estimates.
DISTRIBUTION
They are found in temperate and subarctic waters of the North Atlantic.
RELATION TO HUMANS
The species has never been subject to any commercial hunt. Small scale hunting for food has occurred in Norway, Greenland, Iceland, the Faroe Islands and Canada.
CONSERVATION AND MANAGEMENT
As a widespread and abundant species, with no reported population declines or major threats identified, it receives little management attention.
The species is listed as ‘Least Concern’ in the most recent regional assessment for Europe (2023) on the IUCN Red List. It is listed as ‘Least Concern’ on both the Norwegian and Icelandic national red lists.
LIFESPAN
Maximum reported age for males is 22 years, and 27 years for females.
AVERAGE SIZE
Adults are around 250 cm long and 200-230 kg, with females being slightly shorter and lighter than males.
MIGRATION AND MOVEMENTS
They appear to move throughout a large home range, following the seasonal movements of their fish prey, rather than undertaking specific seasonal migrations.
FEEDING
They have a varied diet, opportunistically feeding on schooling fish and, occasionally, squid and octopus. Their diet also varies with season and location.
General Characteristics
The Atlantic white-sided dolphin (Lagenorhynchus acutus) is a large dolphin found in the temperate and sub-polar waters of the North Atlantic. It has been named for the distinctive light-coloured patches along its sides. The Pacific white-sided dolphin (Lagenorhynchus obliquidens) is a similar dolphin of the same genus found in the northern Pacific Ocean. On the NAMMCO website, “white-sided dolphin” is used to refer only to the Atlantic species.
This dolphin’s beak is short (about 5 cm long) and is not distinctly separated from the melon. The dorsal fin is relatively tall and strongly curved, and can be up to 33 cm high (Reeves et al. 1999). The flippers are long and pointed.
Colouration
The Atlantic white-sided dolphin is more colourful than other species of dolphins. The colouration may, however, be less distinct in juveniles. The upper body is dark grey to black (including the upper jaw, melon, dorsal fin, flippers and flukes), while the belly and lower jaw are white, with the exception of a black patch around the genital slit. In most individuals, there is a black ring around the eye that is connected to the upper jaw by a black line. There is also a very thin black line that connects the eye ring with the outer ear opening. A grey stripe connects the eye and flipper. The sides of the body are light grey, apart from a white patch that extends from below the dorsal fin and continues toward the tail. Some individuals may have two patches – one white and one yellow or yellow-brown. The thick tailstock has a noticeable yellowish band.
Size
Adult dolphins are around 250 cm in length, with males being slightly longer and heavier than females of the same age (Addink et al. 1997). The longest reported dolphin was 282 cm (Reeves et al. 1999). In a survey of 65 stranded Atlantic white-sided dolphins, the longest male found was 267 cm with a weight of 234 kg, while the longest female was 243 cm and a weight of 182 kg (Sergeant et al. 1980).
Misidentification
The Atlantic white-sided dolphin may easily be misidentified as the white-beaked dolphin (L . albirostris) as their ranges overlap considerably. The white-sided dolphin is typically smaller than the white-beaked dolphin, and it has the distinctive white and yellow streaks on its side. The number of teeth also differs, with the white-sided dolphin having more teeth than the white-beaked.
Life History and Ecology
Behaviour
Surface displays
Atlantic white-sided dolphins are quite active at the surface of the sea, often breaching and tail slapping. They are attracted to boats and will ride the bow or stern waves (Weinrich et al. 2001). It is primarily adult or larger dolphins that appear to engage in this behaviour. In numerous observations in US waters between 1984 and 1997, small dolphins or cow-calf pairs were rarely seen riding boat waves (Weinrich et al. 2001). These dolphins have also been observed scavenging in the wake of a fishing trawler (Leopold and Couperus 1995). Other studies, however, have observed adverse reactions to vessels, with dolphins moving away from them (Macleod 2004).
White-sided dolphins are often seen with other cetaceans such as fin, humpback and pilot whales (Reeves et al. 1999) and may form mixed schools with other dolphins. With the larger whales, they have been seen travelling in front, riding the “bow waves” (Weinrich et al. 2001), but they may also be feeding, scavenging fish that the whales bring to the surface. Humpback whales observed in coastal US waters appeared to be disturbed by white-sided dolphins, displaying aggressive activity in their presence (Weinrich et al. 2001).
Social behaviour
Little is known about the social behaviour of this species. Breaching and jumping out of the water seemed to be connected to social contexts, as jumps were more frequently seen when dolphins were part of larger rather than smaller groups (Weinrich et al. 2001). These dolphins also produce various sounds for communication, including squeals, whistles, clicks and buzzes (Reeves et al. 1999, Hamran 2014). Hamran (2014) found clicks to be the dominant sound produced, and noted that overall sound production increased during periods of socialising.
Group size for Atlantic white-sided dolphins varies from several dozen to large herds of several hundred individuals. Groups of 50 to 60 animals are reported to be common in the waters off Newfoundland (Reeves et al. 1999) and in observations off New England, an average group size of 52 animals was recorded (Weinrich et al. 2001). It has been suggested that the dolphins split into smaller groups while feeding and form larger groups for travelling (Gaskin 1992). Some cooperative feeding behaviour has been observed, e.g. a group of dolphins encircling and feeding on a school of sand lance in US waters (Weinrich et al. 2001). Cooperative feeding behaviour has also been observed in these dolphins in Northern Norway (Hamran 2014).
There does not seem to be any kinship relation between dolphins in a particular pod or group. Mirimin et al. (2011) found that the white-sided dolphins sampled in two mass stranding events in Ireland were mainly unrelated to each other. Fernández et al. (2016) also found no evidence of higher levels of kinship within pods of dolphins sampled in the Faroe Islands.
Diving and mobility
White-sided dolphins are highly mobile. A satellite-tagged dolphin in the Gulf of Maine travelled an estimated distance of over 300 km in 64.3 hours (Mate et al. 1994). The same study found that the dolphin spent most of its time (89%) underwater, carrying out many short dives, 76% of which were less than one minute long. A second study of four dolphins outfitted with time-depth recorders found a great deal of variability in dive depth and duration both between and within individuals. The mean dive depths for the 4 individuals ranged from 8.6 m to 40.3 m, and mean dive length ranged from 46 to 296 seconds (Sampson et al. 2012).
These dolphins appear to move throughout a large home range, following the seasonal movements of their fish prey, rather than undertaking specific seasonal migrations (Gaskin 1992).
Strandings
Mass strandings, which can involve from several dozen to over a hundred individuals, are common for this species (Gaskin 1992, Waring et al. 2006). There are some seasonal differences in stranding rates, which are thought to coincide with dolphin distribution at different times of the year. Off New York and Massachusetts, for example, most strandings occur during the winter months (Palka et al. 1997, Craddock et al. 2009), while in the northern UK, the majority of strandings occur during the summer (Reeves et al. 1999).
It is not known why these dolphins strand. Some authors suggest that they are following prey inshore. In the Cape Cod region, dolphins often swim on the high tide into marshes, mudflats, and tidal creeks (Sampson et al. 2012), and so may become stranded once the tide goes out. In a stranding on the west coast of Ireland, the largest animal in the group was found to be seriously ill at the time of stranding, and it was proposed that the rest of the pod may have followed it into shore (Rogan et al. 1997). Other suggestions of why dolphins strand include extreme weather, man-made acoustic disturbance, geomagnetic anomalies and coastal topography. It is possible that the reasons for stranding may be a combination of these various factors, which can also differ between locations.

Life History
From samples taken from 65 stranded white-sided dolphins, length at sexual maturity was found to be from 201 – 222 cm for females, at an age of 6 – 12 years (Sergeant et al. 1980). Males from that study were found to be mature at lengths of 215 – 230 cm from 7 – 11 years old. From by-catch samples taken in the northeastern Atlantic, male dolphins were found to become sexually mature at age 7 or 8 (Neuenhagen et al. 2007). In the same study, mature males were found with quiescent (or dormant) testes, indicating that reproduction occurs seasonally, beginning in February (Neuenhagen et al. 2007). Some female Atlantic white-sided dolphins stranded on the west coast of Ireland in September 1994 had small foetuses present, suggesting that mating took place in late August or early September (Rogan et al. 1997).
Sexually mature female dolphins found stranded on the west coast of Ireland between 1990 and 2006 ranged from 11 – 15 years of age (Mirimin et al. 2011). Examination of their reproductive tracts indicated an interval between births of at least 2 years. This agrees with other studies that have found the calving interval to be 2 to 3 years (Waring et al. 2006).
Births occur during the summer months after a gestation period of 10 – 12 months. The birthing period runs from May to early August, with most births taking place in June and July (Waring et al. 2006). Length at birth is around 110 cm, and the lactation period is about 18 months (Gaskin 1992).
Food and feeding
Atlantic white-sided dolphins have a varied diet, feeding opportunistically on schooling fish such as herring, mackerel and different cod species. Occasionally they also eat squid and octopus (Reeves et al. 1999, Hammond et al. 2008). Most of the information on their diet has been obtained from examinations of the stomach contents of stranded individuals. These examinations indicate that their diet varies by both location and time of year.
Main prey
Atlantic white-sided dolphins stranded on the west coast of Ireland were found to have mainly gadoid fish in their stomachs, as well as Trispoterus sp., herring (Clupea harengus), scad (Trachurus trachurus) and one argentine (Argentina sphyraena)(Rogan et al. 1997). Dolphins caught in fishing trawls in this area were found to have been feeding heavily on mackerel (Scomber scombrus), likely following the schools of these fish as they move inshore to spawn (Couperus 1997).
Blue whiting (Micromesistius poutassou) has also been identified as a prey species in the northeastern Atlantic (Gaskin 1992), as well as tacauds (Trisopterus spp.), horse mackerel (Trachurus trachurus), pilchard (Sardina pilchardus), sand lances (Ammodytes spp.), pollock (Pollachius virens), whiting (Merlangius merlangus), haddock (Melanogrammus aeglefinus), gobies (Gobiidae), and dragonets (Callionymidae) (Craddock et al. 2009). Other oceanic fishes such as silvery pout (Gadiculus argenteus), lanternfishes, and pearlsides (Maurolicus muelleri) have also been found in the diet (Couperus 1997).
In the northwestern Atlantic, a detailed study of the diet of 62 Atlantic white-sided dolphins has been carried out. Thirty-four dolphins were stranded on Cape Cod, while the other 28 were caught by net. Twenty-six different fish species and 3 cephalopod species were identified in the stomachs. The main prey found were silver hake (Merluccius bilinearis), spoonarm octopus (Bathypolypus bairdii), haddock and sand lances (Craddock et al. 2009). The same study found a seasonal variation in the diet. Pelagic Atlantic herring (Clupea harengus) was seen to be the most important prey in summer, but was rare in winter.
Sand lances (Ammodytes spp.) are thought to be an important prey species for white-sided dolphins, although they are rarely found in the stomach contents examined. One possible explanation is that since sand lances are small and have a thin bony structure, they may be digested quickly and therefore be underrepresented in stomach content data (Weinrich et al. 2001).
Predators
White-sided dolphins have few natural predators, with none reported in the literature. The killer whale (Orcinus orca) would likely be one of the few species that could prey on these dolphins.
Health – diseases and parasites
Studies of stranded white-sided dolphins have found various parasites in the digestive tract and other tissues. From dolphins stranded in Maine, USA, two digenes: Pholeter gastrophilus and Oschmarinella laevicaecum, one cestode: Tetrabothrius forsteri, two nematodes: Anisakis sp. and Stenurus globicephalae, and one acanthocephalan: Bolbosoma sp. were identified from the gut (Beverly-Burton 1978). The nematode Stenurus globicephalae is considered a lung worm and is usually found in the respiratory tracts and lungs of dolphins. In this case, it was felt that they had likely been passed up the trachea and been swallowed (Beverly-Burton 1978). Other nematodes considered to be lung worms have been found in white-sided dolphins: Torynurus convolutes in the cranial sinuses (Harris 1979 in Reeves et al. 1999) and Pseudalius inflexis in the bronchi and lungs (Evans 1991 in Reeves et al. 1999).
The digene Pholeter gastrophilus was also found in white-sided dolphins stranded on the Irish coast (Rogan et al. 1997). Other parasites found in this study were Phyllobothrium delphini in the blubber, as well as Pholeter gastrophilus and Monorygma grimaldii in the digestive tract.
Nematodes are a common parasite of these dolphins and have been found in various organs and tissues in white-sided dolphins stranded in various locations throughout their range (e.g. Geraci et al. 1978, Rogan et al. 1997, Powell et al. 2012). In dolphins stranded in Maine, some females were found to have severe infections of the nematode Crassicauda grampicola in their mammary glands, to an extent that it was thought to perhaps interfere with their ability to nurse their young (Geraci et al. 1978).
Dolphins stranded in Massachusetts, USA, were found to be infected with a protozoan parasite Sarcocystis sp. (Protozoa, Apicomplexa) in both skeletal and cardiac muscle tissues (Ewing et al. 2002).
Distribution
Atlantic white-sided dolphins are found in temperate and subarctic waters of the North Atlantic, mainly in the waters of the continental shelf to around the 100 m depth contour (Waring et al. 2006), but also in open oceanic waters. They range in the western Atlantic from southern Greenland to south of Cape Cod, Massachusetts (about 37°N), occasionally being seen as far south as North Carolina (Waring et al. 2006). In 2008, a single white-sided dolphin was found stranded on the coast of South Carolina (Powell et al. 2012).
Northwest Atlantic
Large concentrations of these dolphins are seen in the summer months in US waters, on the continental shelf off Cape Cod and in the southern Gulf of Maine. They are also regularly observed further north, in the lower Bay of Fundy in summer, as well as around Newfoundland and southern Labrador. Sightings during the summer along the Atlantic coast of Nova Scotia, however, are rare (Gaskin 1992). They are found in the Gulf of St. Lawrence and have been spotted in the St. Lawrence River as far west as the Saguenay River (Gaskin 1992).
Northeast Atlantic
In the Northeastern Atlantic, white-sided dolphins are found in the waters between East Greenland, Iceland, the British Isles and western Norway. These dolphins are abundant around the Faroe Islands, and also in the waters between the Faroe and Shetland Islands, especially in late summer and autumn (Reeves et al. 1999). They are sometimes found in Danish waters and the Kattegat, but rarely enter the Baltic Sea (Hammond et al. 2008). This species is common in waters south of Iceland. During one research cruise it was one of four cetacean species commonly observed over the mid-Atlantic ridge (Doksæter et al. 2008). The southern limit of the range in the northeast is the Brittany coast of France, although they have been seen as far south as the Strait of Gibraltar (Hammond et al. 2008).
Habitat
In continental shelf waters, white-sided dolphins appear to prefer areas where there are steep slopes and, in some cases, underwater canyons (Gaskin 1992, Palka et al. 1997, Reeves et al. 1999). Other important factors in their distribution appear to be sea water temperature and salinity, with white-sided dolphins preferring cooler, less saline waters than other species (Selzer and Payne1988, Doksæter et al. 2008). It is not clear whether these factors themselves are important, or rather if they are key influences in determining the presence of the dolphin’s preferred prey, which in turn may affect dolphin distribution. Areas with high sea floor relief, for example, produce local nutrient upwelling, which results in increased productivity and fish abundance.
Changes in the distribution of certain prey species for white-sided dolphins has been suggested as a reason for a habitat shift that was seen in US waters during the 1970s. Prior to that time, white-sided dolphins were found primarily offshore on the continental slope, while white-beaked dolphins were observed mainly on the continental shelf. During that decade, the habitat use between these two species switched, with white-sided dolphins appearing more inshore while white-beaked moved offshore (Waring et al. 2006).
North Atlantic stocks
Three “population units” have been proposed for the northwestern Atlantic white-sided dolphins: the Gulf of St Lawrence, the Gulf of Maine and the Labrador Sea (Palka et al. 1997). This division was based on the distribution of sightings, strandings and incidental takes by fisheries. White-sided dolphins are common in the southern Gulf of Maine and known in the Gulf of St. Lawrence, but there are few sightings and strandings along the Atlantic coast of Nova Scotia (Gaskin 1992), indicating some geographical separation.
There are, however, no other indications of any stock separation for this species. No phenotypic differences were found among the skulls of 228 white-sided dolphins collected from both the eastern and western North Atlantic (Mikkelsen and Lund 1994). Genetic studies also found no detectable differences between dolphins from the westernmost part of the eastern North Atlantic and those from the western North Atlantic (Banguera-Hinestroza et al. 2014). This study did find some differentiation between dolphins sampled east and north of Scotland and samples collected further west, which they felt could indicate a coherent migratory stock in the North Sea. Otherwise, their data suggests a single population across the North Atlantic (Banguera-Hinestroza et al. 2014). A more recent genetic study found no evidence of population differentiation in 43 Atlantic white-sided dolphins sampled throughout their range in the northeast Atlantic (Fernández et al. 2016).
The lack of clearly defined stock units is perhaps a result of the wide pelagic distribution and high mobility of this species.
Current abundance and trends
Atlantic white-sided dolphins are abundant throughout their range, although there are few surveys available to provide current population estimates. This species is difficult to survey for a number of reasons. They are highly mobile and range throughout a wide pelagic habitat, as well as being difficult to identify to the species level from a distance. They are also active at the water’s surface, making many short dives, which means it is hard to estimate group sizes at a distance. Lastly, they appear to react to vessels, with some dolphins being attracted to them to ride the bow or stern waves (Weinrich et al. 2001) and others showing movements away from the vessel (Macleod 2004). Both behaviours affect the results of a population estimate.
Based on the nucleotide diversity observed in white-sided dolphins and an estimated mutation rate, a total population size of between 100,775 and 146,363 has been estimated by Fernández et al. (2016).
Western North Atlantic
In the western North Atlantic, a survey from 1999 gave an estimate of 51,640 (CV=0.38) dolphins in the Gulf of Maine (Waring et al. 2006). This is considered to be the best estimate for this region, as it provided the most complete coverage of the known habitat. Previous estimates had been based on surveys covering only parts of the known distribution.
In the Gulf of St. Lawrence, an estimate of 11,740 (CV=0.47) was made from a 1995 survey. The same study found far fewer dolphins the following year—only 560 (CV=0.89)—possibly because the survey was flown earlier in the year (Kingsley and Reeves 1998).
Central and Eastern North Atlantic
In aerial surveys in Icelandic and adjacent waters carried out as part of the North Atlantic Sightings Surveys (NASS) in 1987, an abundance of 37,622 Atlantic white-sided dolphins (no variance given) was estimated (Sigurjónsson and Vikingsson 1997). An abundance of 20,444 (95% CI: 12,714–32,874) for both white-sided and white-beaked dolphins together was estimated from the 2001 NASS surveys (NAMMCO 2002).
An abundance estimate was generated from the Icelandic and Faroese components of the Trans North Atlantic Sightings Survey (T-NASS) ship surveys conducted in 2007. This resulted in an estimate of 81,008 white-sided dolphins in the area (95% CI = 27,993–234,429) (NAMMCO 2019). From the Icelandic and Faroese shipboard surveys carried out as part of NASS in 2015, an estimate of 130,000 white-sided dolphins was calculated for the area covered, which was a larger area than for the aerial surveys mentioned above (Pike et al. 2019).
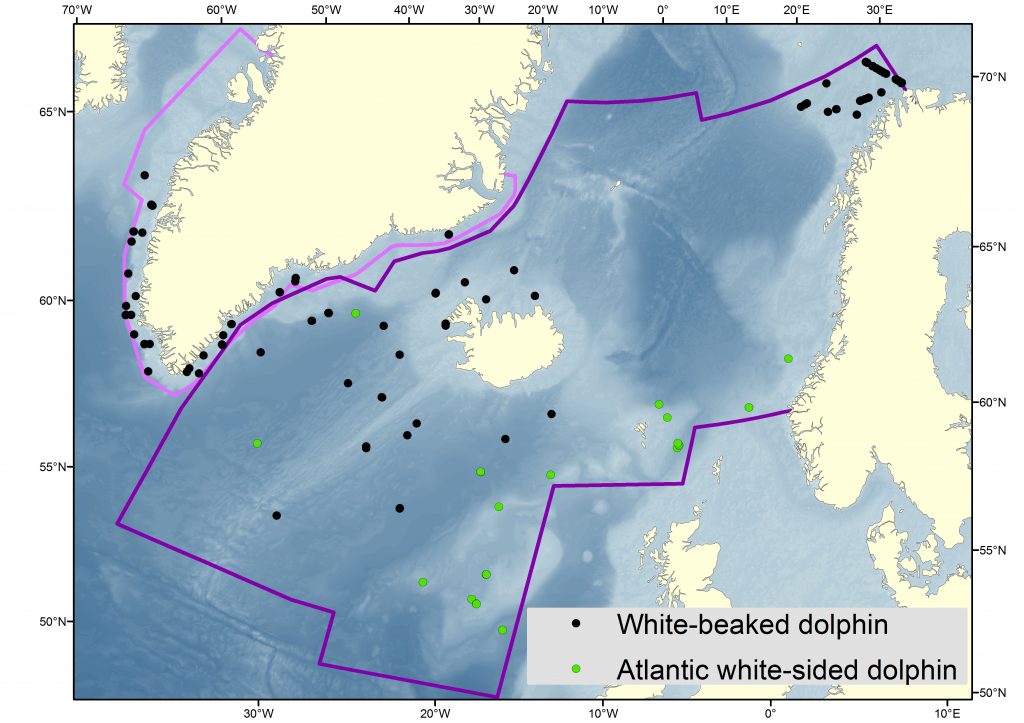
Sightings of white-sided and white-beaked dolphins during NASS 2015. Map: Nils Øien, IMR
A ship survey in the waters between Scotland and the Faroe Islands, carried out in 1998, estimated 21,371 (CV = 0.54) white-sided dolphins to the west of the Outer Hebrides and 74,626 (CV = 0.72) in the Faroe-Shetland Channel (MacLeod 2004).
The Norwegian mosaic surveys, which cover the northern North Sea, the Norwegian Sea, the Greenland Sea and the Barents Sea, have grouped white-beaked and white-sided dolphins together due to a lack of confidence in species identification and therefore abundance estimates from these surveys are provided at the level of the genus (Lagenorhynchus). In 2002-2007, the Norwegian mosaic survey estimated abundance for these delphinids as 218,640 animals (95% CI: 150,330–318,000). In 2008-2013, abundance was estimated at 163,688 animals (95% CI: 112,673–237,800). In 2014-2018, abundance was estimated as being 187,482 animals (95% CI: 112,434–312, 624). All of these abundance estimates, as well as a discussion of the anonymously low estimate in the 2008-2013 cycle, can be found in the 2019 report of the NAMMCO Abundance Estimates Working Group (NAMMCO 2019).
UK & North Sea
From the 1994 SCANS surveys in the ASCOBANS area (waters around the UK, the North Sea and south to the Baltic Sea) a total estimate of 11,760 (CV = 0.26) was made, which included both Atlantic white-sided and white-beaked dolphins (L. albirostris) (Hammond et al. 2002).
The SCANS II survey done in 2005 for the same area also estimated abundance for white-sided and white-beaked dolphins combined, as there was not enough data to make an estimate for white-sided dolphins alone. The estimate for both species for the survey area was 37,981 (CV =0.36) (Hammond 2006). The most recent abundance estimate from the SCANS III survey carried out in the summer of 2016 is 15,510 (CV = .717), for white-sided dolphins alone (Hammond et al. 2017).
Stock status
In any part of their range, population trends for white-sided dolphins will be hard to detect as this species is highly mobile and follows the distribution of their prey. This alone can lead to annual changes in dolphin distribution and abundance in any one area. Overall, the population estimates that have been made show that the white-sided dolphin is generally quite abundant throughout its range.
Two of the projects that might give an indication of possible population trends are the multinational Small Cetacean Abundance in the North Sea and Adjacent Waters (SCANS) survey project and the North Atlantic Sightings Survey (NASS).
SCANS began in 1994 and was repeated in 2005 and 2016. NASS is a series of surveys (using a nearly identical design and methodology) that have been conducted on a regular basis since 1987.
The estimates of dolphin abundance obtained from these surveys are, however, difficult to compare for a number of reasons. White-sided dolphins are not generally the primary target of the surveys. When they are observed, they are generally in large, quickly moving groups. They are also very difficult to identify to the species level from a distance. As a result, they are often reported as, and estimates made for, the genus level, i.e. Lagenorhynchus sp.
For SCANS I and II, the estimates produced were for white-beaked and white-sided dolphins combined, while for SCANS III an estimate was made for white-sided alone. This makes it impossible to detect any specific trend for white-sided dolphins from these surveys.
The NASS surveys also do not reveal any trends, but do indicate that these dolphins have a large population.
Management
The white-sided dolphin is a widespread and abundant species, with no reported population declines or major threats currently identified. As such, it receives little specific management attention.
Hunting is allowed in Greenland year-round for this species. While there are no quotas or catch limits, catch numbers are reported and monitored annually (e.g. Piniarneq 2014). Hunts in the Faroe Islands are also monitored and recorded.
This species is listed in the International Union for the Conservation of Nature (IUCN) red list in the category of “Least Concern” (Hammond et al. 2008).
While not considered endangered, white-sided dolphins are listed in Appendix II of CITES, the Convention on International Trade in Endangered Species of Wild Flora and Fauna, as well as in Appendix II of the Convention on the Conservation of Migratory Species of Wild Animals (CMS). These conventions provide for international cooperation in the management of widely distributed species.
Hunting and utilisation
The Atlantic white-sided dolphin has never been subject to any commercial hunt. Small scale hunting of this species for subsistence has occurred in Norway, Greenland, the Faroe Islands and Canada (Hammond et al. 2008).
Recent catches
Faroe Islands
In the Faroe Islands, white-sided dolphins have been hunted for many centuries. The numbers taken have varied greatly from year to year. In records kept between 1872 and 2003, the numbers of dolphins caught annually ranged from 0–744 (NAMMCO 2003). Catch frequency decreased greatly after 2006, with no reported catches in 2007, 2011, 2012 and 2014-2016.
Canada
There has been occasional hunt of white-sided dolphins in Canada, primarily in Newfoundland and Labrador (Reeves et al. 1999). The numbers taken, however, are not recorded.
Greenland
Hunting of this species for subsistence purposes occurs in Greenland. Catch numbers are reported each year, although data from between 1992 and 2020 are combined catches of white-beaked and Atlantic white-sided dolphins, as there was no Greenlandic name to distinguish between the two species prior to 2020. Between 2006 and 2011, a total of 802 dolphins were taken (Piniarneq 2014). Hunting for dolphins in Greenland is opportunistic, with the numbers taken each year varying from 39 in 2007 to 261 in 2010. The average catch of dolphins between 2011 and 2015 was 160 per year, which was an increase over the previous 5 years (2006-2010) in which the average was 115 per year (NAMMCO 2016). It is not known whether this was due to an increase in the dolphin population around Greenland or if hunters are targeting dolphins more. In recent years (2017-2019), catches have been below the 2011-2016 average.
Catches in NAMMCO member countries since 1992
| Country | Species (common name) | Species (scientific name) | Year or Season | Area or Stock | Catch Total | Quota (if applicable) |
|---|---|---|---|---|---|---|
| Faroe Islands | Atlantic white-sided dolphin | Lagenorhynchus acutus | 2023 | North Atlantic | 10 | |
| Faroe Islands | Atlantic white-sided dolphin | Lagenorhynchus acutus | 2022 | North Atlantic | 0 | |
| Faroe Islands | Atlantic white-sided dolphin | Lagenorhynchus acutus | 2021 | North Atlantic | 1423 | |
| Faroe Islands | Atlantic white-sided dolphin | Lagenorhynchus acutus | 2020 | North Atlantic | 8 | |
| Faroe Islands | Atlantic white-sided dolphin | Lagenorhynchus acutus | 2019 | North Atlantic | 8 | |
| Faroe Islands | Atlantic white-sided dolphin | Lagenorhynchus acutus | 2018 | Faroe Islands | 256 | |
| Faroe Islands | Atlantic white-sided dolphin | Lagenorhynchus acutus | 2017 | Faroe Islands | 488 | |
| Faroe Islands | Atlantic white-sided dolphin | Lagenorhynchus acutus | 2016 | Faroe Islands | 0 | |
| Faroe Islands | Atlantic white-sided dolphin | Lagenorhynchus acutus | 2015 | Faroe Islands | 0 | |
| Faroe Islands | Atlantic white-sided dolphin | Lagenorhynchus acutus | 2014 | Faroe Islands | 0 | |
| Faroe Islands | Atlantic white-sided dolphin | Lagenorhynchus acutus | 2013 | Faroe Islands | 430 | |
| Faroe Islands | Atlantic white-sided dolphin | Lagenorhynchus acutus | 2012 | Faroe Islands | 0 | |
| Faroe Islands | Atlantic white-sided dolphin | Lagenorhynchus acutus | 2011 | Faroe Islands | 0 | |
| Faroe Islands | Atlantic white-sided dolphin | Lagenorhynchus acutus | 2010 | Faroe Islands | 14 | |
| Faroe Islands | Atlantic white-sided dolphin | Lagenorhynchus acutus | 2009 | Faroe Islands | 170 | |
| Faroe Islands | Atlantic white-sided dolphin | Lagenorhynchus acutus | 2008 | Faroe Islands | 1 | |
| Faroe Islands | Atlantic white-sided dolphin | Lagenorhynchus acutus | 2007 | Faroe Islands | 0 | |
| Faroe Islands | Atlantic white-sided dolphin | Lagenorhynchus acutus | 2006 | Faroe Islands | 622 | |
| Faroe Islands | Atlantic white-sided dolphin | Lagenorhynchus acutus | 2005 | Faroe Islands | 312 | |
| Faroe Islands | Atlantic white-sided dolphin | Lagenorhynchus acutus | 2004 | Faroe Islands | 333 | |
| Faroe Islands | Atlantic white-sided dolphin | Lagenorhynchus acutus | 2003 | Faroe Islands | 186 | |
| Faroe Islands | Atlantic white-sided dolphin | Lagenorhynchus acutus | 2002 | Faroe Islands | 773 | |
| Faroe Islands | Atlantic white-sided dolphin | Lagenorhynchus acutus | 2001 | Faroe Islands | 546 | |
| Faroe Islands | Atlantic white-sided dolphin | Lagenorhynchus acutus | 2000 | Faroe Islands | 255 | |
| Faroe Islands | Atlantic white-sided dolphin | Lagenorhynchus acutus | 1999 | Faroe Islands | 0 | |
| Faroe Islands | Atlantic white-sided dolphin | Lagenorhynchus acutus | 1998 | Faroe Islands | 438 | |
| Faroe Islands | Atlantic white-sided dolphin | Lagenorhynchus acutus | 1997 | Faroe Islands | 350 | |
| Faroe Islands | Atlantic white-sided dolphin | Lagenorhynchus acutus | 1996 | Faroe Islands | 152 | |
| Faroe Islands | Atlantic white-sided dolphin | Lagenorhynchus acutus | 1995 | Faroe Islands | 151 | |
| Faroe Islands | Atlantic white-sided dolphin | Lagenorhynchus acutus | 1994 | Faroe Islands | 258 | |
| Faroe Islands | Atlantic white-sided dolphin | Lagenorhynchus acutus | 1993 | Faroe Islands | 377 | |
| Faroe Islands | Atlantic white-sided dolphin | Lagenorhynchus acutus | 1992 | Faroe Islands | 47 | |
This database of reported catches is searchable, meaning you can filter the information by for instance country, species or area. It is also possible to sort it by the different columns, in ascending or descending order, by clicking the column you want to sort by and the associated arrows for the order. By default, 30 entries are shown, but this can be changed in the drop-down menu, where you can decide to show up to 100 entries per page.
Carry-over from previous years are included in the quota numbers, where applicable.
You can find the full catch database with all species here.
For any questions regarding the catch database, please contact the Secretariat at nammco-sec@nammco.no.
Other human impacts
By-catch
White-sided dolphins may become entangled or caught in various types of commercial fishing gear. This species seems to be especially prone to getting caught in pelagic or near surface trawl or drift nets (Gaskin 1992, Reeves et al. 1999). Mortality in fishing gear has been documented off Canada, the United States, the United Kingdom and Ireland (Hammond et al. 2008).
In the Gulf of Maine, it was estimated for the period 1990–1995 that around 120 dolphins were caught each year in the groundfish gillnet fishery, and an average of 58 dolphins per year in the groundfish trawl fishery (Palka et al. 1997). Waring et al. (2006) estimated the average annual fishery-related mortality to the Gulf of Maine stock from commercial fisheries to be much lower, at 38 (CV=0.39) per year.
Off the southwest coast of Ireland, white-sided dolphins are caught in the Dutch pelagic trawl fishery in the late winter and early spring. Numbers of by-caught animals vary greatly from year to year (Couperus 1997). In this area, white-sided dolphins have been observed scavenging in the wake of a trawler (Leopold and Couperus 1995), which may increase their chances of being caught in the fishing gear.
Methods to reduce the by-catch of cetaceans in commercial fisheries have been tried in several areas and fisheries. The use of acoustic signallers (or pingers) on nets, for example, may help reduce by-catch, although the effectiveness of these has not been tested for white-sided dolphins specifically.
Noise/disturbance
Because white-sided dolphins use sound to communicate (Hamran 2014), any noise from human activities may interfere with their behaviour. Some adverse responses to noise have been observed in this species, as has been seen in other dolphin species. During seismic surveys around the UK for example, sighting rates for white-sided dolphins were significantly reduced when airguns were firing compared to when they were not (Stone 2003 in Macleod 2004).
Other potential sources of disturbance could include shipping, oil and gas exploration, or any other human activity occurring in their habitat.
Contaminants
Persistent organic pollutants (POPs) such as PCBs and organochlorine pesticides (e.g. DDT, DDE) and polybrominated diphenyl ether (PBDE) flame retardants have been found in white-sided dolphins sampled throughout their range (McKenzie et al. 1997, Weisbrod et al. 2001, Tuerk et al. 2005, Montie et al. 2009).
The contaminants present in the highest concentrations in white-sided dolphins for all age classes sampled from the northeastern US were PCBs (Tuerk et al. 2005). A previous study had also found high levels of PCBs in dolphins from this region, with levels twice as high as pilot whales from the same area (Weisbrod et al. 2001), although both species had similar levels of pesticides in their tissues. Male dolphins have been found to have higher levels of PCBs in their tissues than females, suggesting that just as in other mammals, females pass some of these contaminants on to their offspring through lactation (Tuerk et al. 2005).
Heavy metals have also been found in white-sided dolphins. For example, in dolphins sampled in the Faroe Islands, cadmium levels were lower than those of pilot whales from the same area but much higher than levels found in dolphin species from more southerly latitudes (Gallien et al. 2001).
As with most other cetaceans, the full range of effects of these pollutants and what “safe” levels of these compounds might be remains largely unknown.
Climate change
If water temperatures increase due to global climate change, this could have an impact on the habitat available for these dolphins and, consequently, their distribution. In some areas, the white-sided dolphin could become displaced by other species, such as the common dolphin, which prefer warmer waters. Other possible consequences of changing water temperatures include redistribution of the dolphin’s prey species, and the introduction of new diseases or parasites.
Research in NAMMCO member countries
NAMMCO member countries work cooperatively on projects such as the North Atlantic Sightings Survey (NASS) to obtain population estimates for white-sided dolphins in member countrys’ waters. Norway has also participated in the multinational Small Cetacean Abundance in the North Sea and Adjacent Waters (SCANS) surveys.
Addink, M., Garcia Hartman, M. and Couperus, B. (1997). A note on life-history parameters of the Atlantic white-sided dolphin (Lagenorhynchus acutus) from animals caught in the Northeastern Atlantic. Report of the International Whaling Commission, 47, 637-640.
Banguera-Hinestroza, E., Evans, P. G. H., Mirimin, L., Reid, R. J., Mikkelsen, B., Couperus, A. S., Deaville, R., Rogan, E. and Hoelzel A. R. (2014). Phylogeography and population dynamics of the white-sided dolphin (Lagenorhynchus acutus) in the North Atlantic. Conservation Genetics, 15(4), 789-802. https://doi.org/10.1007/s10592-014-0578-z
Beverly-Burton, M. (1978). Helminths of the alimentary tract from a stranded herd of the Atlantic white-sided dolphin, Lagenorhynchus acutus. Canadian Journal of Fisheries and Aquatic Sciences, 35(10), 1356-1359. https://doi.org/10.1139/f78-211
Couperus, A. S. (1997). Interactions between Dutch midwater trawl and Atlantic White-sided dolphins (Lagenorhynchus acutus) southwest of Ireland. Journal of Northwest Atlantic Fishery Science, 22, 209-218. https://doi.org/10.2960/J.v22.a16
Craddock, J. E., Polloni, P. T., Hayward, B. et al. (2009). Food habits of Atlantic white-sided dolphins (Lagenorhynchus acutas) off the coast of New England. Fisheries Bulletin, 107, 384-394.
Doksæter, L., Olsen, E., Nøttestad, L. and Fernö, A. (2008). Distribution and feeding ecology of dolphins along the Mid-Atlantic ridge between Iceland and the Azores. Deep-Sea Research II: Topical Studies in Oceanography, 55, 243–253. https://doi.org/10.1016/j.dsr2.2007.09.009
Ewing, R., Zaias, J., Stamper, M. A., Bossart, G. D. and Dubey, J. P. (2002). Prevalence of Sarcocystis sp. in stranded Atlantic white-sided dolphins (Lagenorhynchus acutus). Journal of Wildlife Diseases, 38(2), 291-296. https://www.jwildlifedis.org/doi/10.7589/0090-3558-38.2.291
Fernández, R., Schubert, M., Vargas-Velázquez, A. M., … and Orlando, L. (2016). A genomewide catalogue of single nucleotide polymorphisms in white-beaked and Atlantic white-sided dolphins. Molecular Ecology Resources, 16, 266-276. https://doi.org/10.1111/1755-0998.12427
Gallien I, Caurant F, Bordes M., Bustamante, P., Miramand, Fernandez, B., Quellard, N. and Babin, P. (2001). Cadmium-containing granules in kidney tissue of the Atlantic white-sided dolphin (Lagenorhyncus acutus) off the Faroe Islands. Comparative Biochemistry and Physiology, Part C, 130, 389-395. https://doi.org/10.1016/S1532-0456(01)00265-4
Gaskin, D. E. (1992). Status of the Atlantic white-sided dolphin, Lagenorhynchus acutus, in Canada. Canadian Field-Naturalist, 106, 64-72.
Geraci, J. R., Dailey, M. D. and St. Aubin, D. J. (1978). Parasitic mastitis in the Atlantic white-sided dolphin, Lagenorhynchus acutus, as a probable factor in herd productivity. Canadian Journal of Fisheries and Aquatic Siences, 35(10), 1350-1355. https://doi.org/10.1139/f78-210
Hammond, P. S. (2006). Small Cetaceans in the European Atlantic and North Sea (SCANS II) SCANS II final report. 55 pp.
Hammond, P. S., Lacey, C., Gilles, A. et al. (2017). Estimates of cetacean abundance in European Atlantic waters in summer 2016 from the SCANS-III aerial and shipboard surveys. SCANS III final report. 40 pp.
Hammond, P. S., Bearzi, G., Bjørge, A. et al. (2008). Lagenorhynchus acutus. The IUCN Red List of Threatened Species 2008: e.T11141A3255721. http://dx.doi.org/10.2305/IUCN.UK.2008.RLTS.T11141A3255721.en. Downloaded 14 November 2017.
Hammond, P. S., Berggren, P., Benke H., Borchers, D. L., Collet, A., Heide‐Jørgensen, M. P., Heimlich, S., Hiby, A. R., Leopold M. F. and Øien N. (2002). Abundance of harbour porpoise and other cetaceans in the North Sea and adjacent waters. Journal of Applied Ecology, 39, 361-376. https://doi.org/10.1046/j.1365-2664.2002.00713.x
Hamran, E. T. (2014). Distribution and vocal behavior of Atlantic white-sided dolphins (Lagenorhynchus acutus) in northern Norway. MS Thesis, University of Nordland.
Kingsley, M. C. S. and Reeves, R. R. (1998). Aerial surveys of cetaceans in the Gulf of St. Lawrence in 1995 and 1996. Canadian Journal of Zoology, 76, 1529-1550. https://doi.org/10.1139/z98-054
Leopold, M. F. and Couperus, A. S. (1995). Sightings of Atlantic white-sided dolphins Lagenorhynchus acutus near the south-eastern limit of the known range in the North-East Atlantic. Lutra, 38, 77-80.
Macleod, K. (2004). Abundance of Atlantic white-sided dolphin (Lagenorhynchus acutus) during summer off northwest Scotland. Journal of Cetacean Research and Management, 6(1), 33-40. https://archive.iwc.int/pages/search.php?search=%21collection15&k=
Mate, B. R., Stafford, K. M., Nawojchik, R.and Lawrence Dunn, J. (1994). Movements and dive behavior of a satellite-monitored Atlantic white-sided dolphin (Lagenorhynchus acutus) in the Gulf of Maine. Marine Mammal Science, 10, 116-121. https://doi.org/10.1111/j.1748-7692.1994.tb00398.x
McKenzie, C., Rogan, E., Reid, R. J. and Wells, D. E. (1997). Concentrations and patterns of organic contaminants in Atlantic white-sided dolphins (Lagenorhynchus acutus) from Irish and Scottish coastal waters. Environmental Pollution, 98, 15-27. https://doi.org/10.1016/S0269-7491(97)00109-7
Mikkelsen, A. M. H. and Lund, A. (1994). Intraspecific variation in the dolphins Lagenorhynchus albirostris and L. acutus (Mammalia, Cetacea) in metrical and nonmetrical skull characters, with remarks on occurrence. Journal of Zoology, 234, 289-299. https://doi.org/10.1111/j.1469-7998.1994.tb06076.x
Mirimin, L., Banguera-Hinestroza, E., Dillane, E., Hoelzel, A. R., Cross, T. F. and Rogan, E. (2011). Insights into genetic diversity, parentage, and group composition of Atlantic white-sided dolphins (Lagenorhynchus acutus) off the West of Ireland based on nuclear and mitochondrial genetic markers. Journal of Heredity, 102(1), 79–87. https://doi.org/10.1093/jhered/esq106
Montie, E. W., Reddy, C. M., Gebbink, W. A., Touhey, K. E., Hahn, M. E. and Letcher, R. J. (2009). Organohalogen contaminants and metabolites in cerebrospinal fluid and cerebellum gray matter in short-beaked common dolphins and Atlantic white-sided dolphins from the western North Atlantic. Environmental Pollution, 157, 2345-2358. https://doi.org/10.1016/j.envpol.2009.03.024
North Atlantic Marine Mammal Commission (NAMMCO). (2002). NAMMCO Annual Report 2002. NAMMCO, Tromsø, Norway, 357 pp. https://nammco.no/topics/annual-reports/
North Atlantic Marine Mammal Commission (NAMMCO). (2003). NAMMCO Annual Report 2003. NAMMCO, Tromsø, Norway, 373 pp. https://nammco.no/topics/annual-reports/
North Atlantic Marine Mammal Commission (NAMMCO). (2016). NAMMCO Annual Report 2016. NAMMCO, Tromsø, Norway, 363 pp. https://nammco.no/topics/annual-reports/
North Atlantic Marine Mammal Commission (NAMMCO). (2019). Report of the Abundance Estimates Working Group, October, Tromsø, Norway. https://nammco.no/topics/abundance_estimates_reports/
Neuenhagen, C., García Hartmann, M. and Greven, H. (2007). Histology and morphometrics of testes of the white-sided dolphin (Lagenorhynchus acutus) in bycatch samples from the Northeastern Atlantic. Mammalian Biology, 72(5), 283-298. https://doi.org/10.1016/j.mambio.2006.10.008
Palka, D., Read, A. and Potter, C. (1997). Summary of knowledge of white-sided dolphins (Lagenorhynchus acutus) from US and Canadian Atlantic waters. Report of the International Whaling Commission, 47, 729–734.
Pike, D.G., Gunnlaugsson, T., Mikkelsen, B., Halldórsson, S.D. & Víkingsson, G.A.(2019). Estimates of the abundance of cetaceans in the central North Atlantic cased on the NASS Icelandic and Faroese shipboard surveys conducted in 2015. NAMMCO Scientific Publications 11. https://doi.org/10.7557/3.4941.
Piniarneq. (2014). Jagtinformation og fangstregistrering. Government of Greenland, Nuuk, Greenland.
Powell, J. W. B., Rotstein, D. S. and Mc Fee, W. E. (2012). First records of the melon-headed whale (Peponocephala electra) and the Atlantic white-sided dolphin (Lagenorhynchus acutus) in South Carolina. Southeastern Naturalist, 11(1), 23-34. https://doi.org/10.1656/058.011.0102
Rogan, E., Baker, J. R., Jepson, P. D., Berrow, S. and Kiely, O. (1997). A mass stranding of white-sided dolphins (Lagenorhynchus acutus) in Ireland: biological and pathological studies. Journal of Zoology, 242(2),217-227. https://doi.org/10.1111/j.1469-7998.1997.tb05798.x
Reeves, R. R., Smeenk, C., Brownell, R. L. Jr. and Kinze, C. C. (1999). Atlantic white-sided dolphin Lagenorhynchus acutus (Gray, 1828). In S. H. Ridgway and R. Harrison (eds.), Handbook of marine mammals, Vol. 6: The second book of dolphins and the porpoises, 31-56. Academic Press.
Sampson, K., Merigo, C., Lagueux, K., … and Innis, C. (2012). Clinical assessment and postrelease monitoring of 11 mass stranded dolphins on Cape Cod, Massachusetts. Marine Mammal Science, 28(4), 404-425. https://doi.org/10.1111/j.1748-7692.2011.00547.x
Selzer, L. A. and Payne, P. M. (1988). The distribution of white-sided (Lagenorhynchus acutus) and common dolphins (Delphinus delphis) vs. environmental features of the continental shelf of the northeastern United States. Marine Mammal Science, 4(2), 141-53. https://doi.org/10.1111/j.1748-7692.1988.tb00194.x
Sergeant, D. E., St. Aubin, D. J. and Geraci, J. R. (1980). Life history and northwest Atlantic status of the Atlantic whitesided dolphin, Lagenorhynchus acutus. Cetology, 37, 1-12.
Sharpe, M. & Berggren, P. 2023. Lagenorhynchus acutus (Europe assessment). The IUCN Red List of Threatened Species 2023: e.T11141A219011226. Accessed on 19 December 2023.
Sigurjónsson, J. and Víkingsson, G. A. (1997). Seasonal abundance of and estimated food consumption by cetaceans in Icelandic and adjacent waters. Journal of Northwest Atlantic Fishery Science, 22, 271-287. https://doi.org/10.2960/J.v22.a20
Tuerk, K. S., Kucklick, J. R., McFee, W. E., Pugh, R. S. and Becker, P. R. (2005). Factors influencing persistent organic pollutant concentrations in the Atlantic white-sided dolphin (Lagenorhynchus acutus). Environmental Toxicology and Chemsitry, 24(5), 1079-1087. https://doi.org/10.1897/04-120R.1
Waring, G. T., Josephson, E., Fairfield, C. P. et al. (eds.) (2006). U.S. Atlantic and Gulf of Mexico marine mammal stock assessments – 2005. NOAA Technical Memorandum NMFS-NE – 194 346 pp.
Weinrich, M. T., Belt, C. R. and Morin, D. (2001). Behavior and ecology of the Atlantic white-sided dolphin (Lagenorhynchus acutus) in coastal New England waters. Marine Mammal Science, 17, 231-248. https://doi.org/10.1111/j.1748-7692.2001.tb01268.x
Weisbrod, A. V., Shea, D., Moore, M. J., Stegeman, J. J.(2001). Species, tissue and gender-related organochlorine bioaccumulation in white-sided dolphins, pilot whales and their common prey in the northwest Atlantic. Marine Environmental Research, 51(1), 29-50. https://doi.org/10.1016/S0141-1136(00)00032-5















