Harp Seal
Harp Seal
Latest update: July 2021
The harp seal is a medium-sized, migratory seal widely found over the continental shelf regions of the North Atlantic ocean. It is the most abundant pinniped in the North Atlantic (Kovacs 2015) and is named for the distinctive black band found on the back of adult males. This band forms a harp shape while the rest of the body is white or a pale grey colour. The head and tail are black or dark brown and the anterior flippers and belly are whitish. The “harp” is often not as well defined in adult females, which commonly have light coats with dark spots.
DISTRIBUTION
Harp seals are found in the continental shelf regions of the North Atlantic Ocean.
ABUNDANCE
In 2019, the estimated abundance of harp seals was in the order of:
Northwest Atlantic: 7.4 million
Greenland Sea: 426, 800
Barents Sea / White Sea: 1.49 million
RELATION TO HUMANS
Harp seals are hunted by indigenous communities throughout their range. In Canada and Norway there are also commercial hunts.
CONSERVATION AND MANAGEMENT
Scientific advice on stock status and sustainable catch scenarios is generated through the International Council for Exploration of the Sea (ICES), the Northwest Atlantic Fisheries Organisation (NAFO) and NAMMCO Joint Working Group on Harp and Hooded Seals.
In the most recent assessment (2015) the species is listed as ‘Least Concern’ on the global IUCN Red List, as well as on the Norwegian national red list.


Harp seal female with pup. © Ann Harvey
Scientific name: Pagophilus groenlandicus *
Faroese: Grønlandskópur
Greenlandic: Aataaq
Icelandic: Vôðuselur
Norwegian: Grønlandssel
Danish: Grønlandssæl
English: Harp seal, Greenland seal, Saddle seal, Saddleback seal
* Note: The scientific name for the harp seal has changed frequently in the past. The generic names Phoca, Pagophilis and Pagophoca, and the specific names groenlandicus and groenlandica have all been used. Pagophilus groenlandicus is the name currently recognized by the Society of Marine Mammalogy (Committee on Taxonomy 2016).
LIFESPAN
Can be over 30 years old
SIZE
Male and female adults similar in size, averaging 1.6 m in length and 130 kg in weight
FEEDING
Wide range of fish and invertebrate prey. Krill, amphipods, polar cod, cod and capelin important prey species
PRODUCTIVITY
Sexual maturity between 4-8 years, then one pup each year
MIGRATION AND MOVEMENTS
Breeding on sea ice, then feeding in open water, return to breeding grounds on forming pack ice in autumn
General Characteristics
Newborn harp seals have a long-haired white coat, which is moulted after weaning to a silver-coloured coat with a few dark spots, called the “beater” pelt. They retain this coat for the rest of their first year. At the next moult, the pelt becomes more spotted and the seal is termed a “bedlamer”. As the seal gets older, the spots become more numerous but will generally disappear altogether once the seal reaches sexual maturity (Richard 2001). Some females, however, will retain spots throughout their lives.
Male and female harp seals are similar in size, with adults averaging 1.6 m in length and 130 kg in weight prior to the breeding season (DFO 2016a). Adults can be up to 195 cm long and weigh as much as 182kg (Richard 2001). A newborn pup will be around 85cm long and weigh about 11kg. Just after weaning (about 2 weeks or less) the pups will have grown to around 115cm and 33kg (Richard 2001, Sergeant 1991).
Harp seals are relatively long-lived and can live to be 30 years old (Sergeant 1991, Kovacs 2015). Both sexes mature when they are between 4 and 8 years old.
BEHAVIOUR
Harps seals are a social species and are rarely seen alone after the first year of life (Kovacs and Lydersen 2016, Andrianov 2014, Sergeant 1991). They travel in groups in the water and also haul out together on the ice.
Harp seals migrate annually, northwards in summer and southwards in winter, following the development and retreat of the pack ice. These seals are often seen feeding along the ice edge throughout the year (Stenson et al. 2016).
Reproduction
Harp seals are very dependent on pack ice, and their genus name refers to this “ice-loving” habit. They use drifting pack ice for whelping (pupping), to nurse their young and to moult. Harp seals give birth in the spring, sometime during February – March, depending on their location.
The mother-pup bond is strong, and females appear to recognize their own pups by both smell and sound (Sergeant 1991). Newborns are nursed for only 10 to 12 days (Stenson et al. 2016). The pups quickly gain weight, up to 20 kg over the nursing period, as harp seal milk has approximately 12 times the fat and 25 times the protein of cows’ milk (Sergeant 1991). Weight of the pups at birth are around 11 kg and at weaning average 34 kg (Sergeant 1991).
After weaning, the adults mate and disperse. Although mating occurs shortly after the pups are weaned, harp seals undergo embryonic diapause (delayed implantation) with implantation occurring in late July or early August (Stenson et al. 2016).
LIFE HISTORY
Both male and female harp seals reach sexual maturity between 4 and 8 years of age. Most animals that reach sexual maturity live to over 20 years (Kovacs 2015) and the maximum life span of a harp seal is approximately 30 years.
Reproduction
Time of pupping
Harp seals give birth in the spring – sometime during February-March depending on their location. For the Northwest Atlantic population, pupping occurs in late February and early March on the pack ice off southern Labrador and northeastern Newfoundland (“the Front”), or in the Gulf of St Lawrence (“the Gulf”). Approximately two-thirds of the pupping occurs at the Front (Stenson et al. 2014) and is highly synchronized with over 90% of the pupping occurring between 6th and 12th March (Stenson et al. 2016). In the Gulf, pupping begins slightly earlier, with 50% of the births occurring by 1st March (Stenson and Hammill 2012).
In the Barents Sea/White Sea, the majority of harp seal females whelp between 25th February and 4th March, but newborns and fresh placental remains have been observed as late as 12th March (Potelov et al. 2003). The Greenland Sea/Jan Mayen population has the latest whelping season, with an average birth date between 18th and 20th March (Sergeant 1991).
Pup development
The mother-pup bond is strong and females appear to recognize their own pups by both smell and sound (Sergeant 1991). Newborns are nursed for only 10 to 12 days (Stenson et al. 2016). The pups quickly gain weight, up to 20 kg over the nursing period, as harp seal milk has approximately 12 times the fat and 25 times the protein of cows’ milk (Sergeant 1991). The weight of the pups at birth is around 11 kg, with an average weight at weaning of 34 kg (Sergeant 1991).
After weaning, the adults mate and disperse. Although mating occurs shortly after the pups are weaned, harp seals undergo embryonic diapause (delayed implantation) for 3-4 months with implantation occurring in late July or early August (Stenson et al. 2016, Stewart et al. 1989). This results in the gestation period lasting almost a full year.
FOOD AND FEEDING
Harp seals consume a wide range of prey, which varies by season along their migration routes. Their recorded diet includes 67 species of fish and 70 species of invertebrates (Kovacs 2015).
The first food for juvenile harp seals after weaning tends to be pelagic crustaceans, especially Euphausiids (krill; Thyanoessa spp.) and pelagic amphipods (Parathemisto spp.). Once the seals are older and able to dive deeper, benthic crustaceans, cephalopods and fish are eaten (Folkow et al. 2004,). Harp seals are opportunistic feeders and take many different species of fish.
Capelin (Mallotus villosus) is the most important species taken in the subarctic while polar cod (Boreogadus saida) is the most important in the Arctic (Sergeant 1991).
Seasonal variation
Adult harp seals feed very little during the periods of breeding and moulting (Folkow et al. 2004). This means that when they arrive in their northern feeding grounds in spring, they are extremely lean. During the summer months they target the most lipid-rich prey such as krill, amphipods and polar cod, and so gain considerable fat reserves, which they deposit in their blubber layer (Lindstrøm et al. 2013).
In the Barents Sea, late summer and autumn appear to be the periods of the most intense feeding. For harp seals sampled in the northern Barents Sea during this period, krill was the most important prey group (63 % of the diet) followed by polar cod (16 %) and other fish species (10 %) (Lindstrøm et al. 2013).
Variation by area
Capelin was found to be the dominant food in southwest and central-west Greenland, with crustaceans, specially euphausids and amphipods, as other important food items, especially for young seals (Sergeant 1991). In north and northwest Greenland, polar cod was seen to be the dominant food species, with Arctic cod (Arctogadus glacialis) also taken, together with pelagic euphausiids and amphipods (Parathemisto spp.)
In southwest Greenland a large variety of fish, including large benthic species such as gadoids and flatfish were taken, as well as prawns (Pandalus borealis) and squid (Gonatus fabricii) (Sergeant 1991).
Off Newfoundland, harp seals eat capelin and Arctic cod, and off Labrador they eat Arctic cod and Atlantic herring (Clupea harengus). In the Gulf of St Lawrence, harp seals consume capelin, herring, Atlantic cod, Arctic cod and redfish (Sebastes sp.) (Kovacs 2015).
PREDATORS
In the southern portions of the harp seal’s range, sharks and killer whales are the dominant predators, while in Arctic locations polar bears are probably the greatest threat (Kovacs and Lydersen 2016). Off the Labrador coast and in Davis Strait, harp seals have been found to be an important component in the diet of polar bears. The stomach contents of killer whales caught in southeast Greenland indicate that harp seal pups are an important part of their diet (ICES 2013).
PARASITES
Harp seals may be a vector of the codworm /sealworm (Pseudoterranova decipiens), a parasitic nematode found in groundfish for which seals are the final host. This worm, while not a health hazard to humans, does result in economic losses to some commercial fisheries, as it must be removed during processing. However, grey seals, not harp seals, appear to be the major source of this parasite (DFO 2016b).
STOCK DISTRIBUTION
Three distinct populations of the harp seal are found in the North Atlantic and Arctic Oceans. Based on their whelping (pupping) sites, these populations are those of: the Northwest Atlantic, the Northeast Atlantic or Greenland Sea, and the Barents Sea/White Sea. Each of these groups has some distinct morphological, genetic and behavioural characteristics. The Northwest Atlantic population is further divided into two major herds: the “Front” herd that whelps off the coast of northern Newfoundland and southern Labrador, and the “Gulf” herd that whelps in the southern Gulf of St. Lawrence.
There is some evidence that an additional whelping patch may be found off southern Greenland, at least in some years (Rosing-Asvid 2007). The Greenland Sea population breeds north of Jan Mayen Island in pack ice areas known as the ‘‘West Ice,’’ and the Barents Sea / White Sea population breeds in areas of pack ice known as the ‘‘East Ice’’ in the White Sea/southeastern Barents Sea (Frie et al. 2003).
Prehistorically, harp seals were also found in the Baltic Sea, but they have since been extirpated from that area (Kovacs 2015).
 HABITAT
HABITAT
Harp seals are very dependent on pack ice, which they use to haul out, to give birth and nurse their young, and to moult. Harp seals rarely haul out on land (Hammill and Stenson 2014). The ice chosen by females at the beginning of the pupping season is crucial because the mobility of the newborn pup is very restricted. Harp seal pups need ice that is stable for a long enough time for them to develop their insulating blubber layer and to rest.
Harp seals whelp on first year ice (i.e. ice from not more than one winter, with a thickness of 30 cm or greater) or grey-white ice (15-30 cm thick) of 6/10 or more areal coverage, which is usually the thickest ice available in their traditional pupping areas (Bajzak et al. 2011, Sergeant 1991). Where possible, the females appear to choose ice pans that are large and thick enough to persist for some time and not be destroyed by storms, but at the same time, not so large that they cannot easily enter the water during the lactation period (Hammill and Stenson 2014).
Harp seals are relatively shallow divers when they are near the pack ice. Seals satellite-tagged in the Greenland Sea stayed close to the edge of the pack ice during the spring moult, usually diving to less than 100 m. However in July they moved into the Barents Sea where they dove to depths of 400m, with one tagged seal diving to 568m (Folkow et al. 2004).
MIGRATIONS
Harp seals undertake an annual cycle of feeding, reproduction, and migration. They can cover very large distances in short periods of time. One tagged female travelled a distance of more than 12,000 km in 298 tracking days, amounting to a daily swimming distance of around 40 km (Folkow et al. 2004).
Northwest Atlantic
After the spring breeding, Northwest Atlantic harp seals follow the retreating pack ice up the coast of Labrador. Some will travel into Hudson Bay and around Baffin Island, with the rest travelling up either the Canadian or Greenlandic side of Davis Strait (Kovacs 2015, Stenson et al. 2016). Along the West Greenland coast, the prevalence of harp seals has increased remarkably since the 1980s, particularly in southwest Greenland (Rosing-Asvid 2008). Large groups of seals are found in the west Greenland fjords from mid – late May to October.
Harp seals begin the return trip to their breeding grounds in late autumn as the pack ice develops, making their round-trip migration in some cases more than 5,000 km (Lavigne and Kovacs 1988). Studies of tagged seals have shown that they have a high degree of site fidelity to their original breeding and moulting areas (Frie et al. 2003).
Northeast Atlantic and Barents Sea/White Sea
The Northeast Atlantic (Greenland Sea) and Barents Sea/White Sea groups also migrate northward in the spring and mix in the Barents Sea (Andrianov 2014, Folkow et al. 2004), reaching at least to Franz Joseph Land and Svalbard. Depending on ice conditions, some harp seals may range up to between 82° and 85° north (Kovacs 2015). Around Svalbard, they are most commonly encountered during the summer on the north and east coasts of Spitsbergen. However, they may also be found in Svalbard’s fjords during the spring and autumn. Their range varies from year to year depending on ice conditions (Kovacs and Lydersen 2016). Harp seals remain in the Barents Sea until late autumn, when the seals from the Northeast Atlantic return to the waters off the coast of East Greenland and the White Sea stock returns to the White Sea.
During this annual cycle of migrations, these seals spend much of their time in open water, with an annual distribution that appears to be closely linked to the distribution of concentrations of capelin (Mallotus villosus) (Folkow et al. 2004).
In September 2008, an adult male harp seal was found stranded near Motril, Granada, Spain, inside the Mediterranean Sea (Bellido et al. 2009). This sighting is the southernmost known report for the harp seal worldwide.
NORTH ATLANTIC STOCKS
Three distinct populations of the harp seal are found in the north Atlantic and Arctic Oceans. Based on their whelping (pupping) sites these are the populations of: the Northwest Atlantic, the Northeast Atlantic or Greenland Sea, and the Barents Sea/White Sea. While there may be limited mixing between the populations, they have been found to be genetically distinguishable (Carr et al. 2015).
The Northwest Atlantic population is further divided into two major herds: the “Front” herd that whelps off the coast of northern Newfoundland and southern Labrador, and the “Gulf” herd that whelps in the southern Gulf of St. Lawrence. There is some evidence that an additional whelping patch may be found off southern Greenland, at least in some years (Rosing-Asvid 2007).
The Northeast Atlantic or Greenland Sea population whelps north of Jan Mayen Island in pack ice areas known as the ‘‘West Ice’’ , and the Barents Sea/White Sea population whelps in the White Sea or southeastern Barents Sea areas of pack ice known as the ‘‘East Ice’’ (Frie et al. 2003).
COUNTING HARP SEALS
The total number of harp seals would be nearly impossible to count directly because the seals are widely distributed across the north Atlantic and Arctic Oceans at most times of the year, where they spend much of their time underwater. While they do congregate during the whelping season, not all of the population is present at the surface at any one time or place. Therefore, direct surveys of the whelping grounds that count seal pups on the ice are used in combination with population models to estimate the total number of harp seals.
Harp seal surveys use a combination of visual and photographic methods (Hammill et al. 2014, 2015, Øigard et al. 2014). Visual reconnaissance surveys, using helicopters and fixed wing aircraft, are used to locate and map the whelping patches. Photographic survey transects are then flown over the patches. Pups are counted on the photos by experienced readers, and the counts are verified by repeated readings. Software-based detection methods using artificial intelligence (deep learning) for image analysis is also currently in development and being trialed. Currently, a semi-automatic approach with validation from a reader seems most feasible (e.g. using artificial intelligence software to discard images showing no seals but a reader to make positive identifications).
The pups are classified by “stages” according to their apparent age, from newborn to weaned pup. These data are then used in combination with information from tagged pups to estimate the proportion of pups that are on the ice, and not in the water, and thus “available” to be seen at any one time. The corrected pup density on the photographic transects are applied to the entire survey area using standard strip transect methodology.
Estimates of pup production are extended to estimates of the total population using population models. These models incorporate data on pregnancy rate at age, natural mortality and catch at age to calculate the number of adult seals that would produce the number of pups observed in the survey (Hammill et al. 2014, 2015, Øigard et al. 2014).
NORTHWEST ATLANTIC STOCK
The Northwest Atlantic harp seal population is near the highest levels observed since monitoring began over 60 years ago, with an estimated 7.4 million animals (Hammill et al. 2014). This is a large increase from the fewer than 1.5 million seals that were estimated to make up this population in the early 1970s (Stenson et al. 2016). The population appears to be relatively stable, with little change in abundance over the last decade although an overall decline in productivity of the herd has been observed (Hammill et al. 2014). Although the Joint ICES/NAFO/NAMMCO Working Group on Harp and Hooded Seals performed assessments in 2016 and 2019, no new information on abundance was presented.
Until the late 1970s, 85% of the mature females were pregnant each year (Stenson et al. 2016). Since then pup production has become highly variable, but with an overall declining trend (ICES 2019). Annual pregnancy rates have varied from 40 to 75% (Stenson et al. 2011).
Declines in the productivity are likely linked to density-dependent effects through resource limitation acting on females, as well as short-term fluctuations in environmental conditions, particularly ice cover. These conditions are expected to continue, at least in the near term, and so productivity of this stock will likely remain relatively low (Hammill et al. 2014). A largely unregulated hunt of harp seals in Greenland will also have an impact on future trends for this population, as will any changes in ice conditions in the whelping areas (Hammill et al. 2011, 2014).
GREENLAND SEA STOCK
Population numbers for this stock have varied considerably over the past several decades. In the early 1960s, a decline in this population to around 500,000 seals was observed (Johnston et al. 2012), but recovered to approximately 800,000 by 1978. After this time, survival of newborn harp seals in this area declined and remained low until the early 1990s (Johnston et al. 2012). The population was estimated to be 296,000 in 1994 (Kovacs 2015). The population increased to approximately 348,000 seals by 2003 and in 2013 was estimated to be 627,000 (ICES 2013, Øigård et al. 2014). The 2019 assessment of the Joint ICES/NAFO/NAMMCO Working Group on Harp and Hooded Seals estimated abundance of harp seals in the Greenland Sea as being 426,808 animals (95% CI: 313,005 – 540,612).
BARENTS SEA/WHITE SEA STOCK
The White Sea/Barents Sea harp seal stock population appears to be recovering from historical low numbers seen in the 1960s (Skaug et al. 2007, ICES 2013). The population was believed to have reached about 1 million (producing about 200,000 pups yearly) by 1980, and was increasing at a rate of about 5% per year (Sergeant 1991). More recently, reductions in pup production and declines in body condition have been documented in this stock, and are thought to be due to environmental change linked to warmer water and poor sea ice conditions (Kovacs 2015).
An estimate produced in 2013 using data from several pup counts done between 1998 and 2010 showed the population of this stock to be around 1.4 million seals, which is thought to be about 83% of the historical maximum population size observed/estimated (ICES 2013). The 2019 assessment of the Joint ICES/NAFO/NAMMCO Working Group on Harp and Hooded Seals estimated abundance of harp seals in the Barents Sea/White Sea as being 1,497,190 animals (95% CI: 1,292,939 – 1,701,440).
STOCK STATUS
Assigning a “status” to a particular stock of animals is a complex and sometimes controversial exercise that requires, among other things, some knowledge of the historical abundance trend of the stock, an assessment of the reliability of that knowledge, and clearly stated management/conservation goals with respect to the stock. There is no universal definition of stock status that is used by all organizations and countries. It is therefore important to clearly define how stock status is being interpreted before any description is given.
The management framework
A management framework for harp and hooded seals that clearly defines stock status has been adopted by NAMMCO member countries and by Canada (NAMMCO 2004a, 2005, Hammill and Stenson 2007). The framework distinguishes between “data rich” and “data poor” stocks based on defined criteria regarding the quality and age of the available information on abundance and biological parameters. For example, data rich stocks must have at least five abundance estimates spanning a period of 10-15 years with surveys separated by 2-5 years, and the most recent abundance estimate should be no more than five years old. Since there will be more uncertainty about the status of data poor stocks, the criteria used to assign status are also different for these stocks.
Data rich stocks
For data rich stocks, the agreed framework establishes numerical population reference levels, the most important of which are the precautionary level and the limit level. For harp seals, the precautionary level is set at 70% of the maximum stock size observed (Nmax) and therefore called N70. The limit level is set at 30% of the maximum stock size observed (N30).
Stocks above N70 are of least conservation concern and management goals can be set to increase, stabilise or decrease the population as long as it stays above N70. If a stock is below N30, the stock is considered to be of greatest conservation concern. Accordingly, no catch can be allowed and management must have the goal of increasing stock size to above N70. Other reference levels may be set between these two, which may allow for limited hunt but always with the goal of increasing stock size to above N70.
Data poor stocks
For data poor stocks, only a limit level (below which no catch is allowed) is required. In addition, catch levels must be set in a more precautionary way to allow the population to increase towards Nmax. Data poor stocks can become data rich as additional surveys are done and more information about the stock becomes available.
Other ways of classifying stocks
Other organizations classify the status of stocks in different ways. For example, the International Union for Conservation of Nature and Natural Resources (IUCN) uses specific criteria to assign species to one of nine classifications ranging from “Least Concern” to “Vulnerable” to “Extinct”.
NORTHWEST ATLANTIC STOCK
This population was overexploited by Newfoundland hunters through the 1800s. The highest level of catches were reported in the early to mid-1800s with an average of over 470,000 seal skins exported annually from Newfoundland between 1840 and 1850 (Stenson 2014). In the early 20th century, catches declined significantly, to a low of 15,300 per year during World War II, and the stock recovered.
New exploitation by Canada and by Norway after 1946 reduced the Northwest Atlantic population again. The total population in 1952 was estimated at 2.3 million animals (95% CI: 2,200,000 – 2,400,000). In the mid 1960s, adult females became protected on the breeding grounds and Norway was excluded from sealing in Canadian waters (Kovacs 2015). In 1971, the population reached a minimum of around 1.1 million animals (Hammill et al. 2011, 2015). Quotas to limit the hunt were introduced at that time, as well as a closure of the Gulf of St. Lawrence to hunting from large vessels. This saw the population increase, reaching some 7.8 million animals in 2008 (Hammill et al. 2015).
Today
Currently, this population is estimated at around 7.4 million animals. This is below the estimated pre-hunt level for these herds of 12 million seals (Hammill et al. 2014).
This stock of harp seals is managed under a five-year Integrated Fisheries Management Plan for Atlantic Seals (DFO 2016b). The main tool used to evaluate the status of this stock is an aerial survey to count the number of pups born in the whelping areas. Such a survey has been flown every 4-5 years since 1990 (DFO 2016c). Additional information is collected annually, including data on age-specific reproductive rates, catch levels in the Canadian and Greenland hunts, and mortality estimates of young of the year. A detailed assessment of the stock is completed approximately every five years, with annual updates providing catch advice to fisheries managers (DFO 2016b).
Part of the Integrated Fisheries Management Plan involves setting a quota or Total Allowable Catch (TAC) for the commercial seal hunt. Prior to 2006, the TAC was based upon a management plan that allowed for a total of 975,000 seals over 3 years with a maximum of 350,000 in any one year. After 2005, TACs were set annually to ensure that the population did not decline below the precautionary reference level (“N70” or 70% of the maximum population size) within a 15 year period. The TAC for the 2016 season was set at 400,000 animals (DFO 2016a) and since then quotas have remained the same. After 2017, the TAC has not actually been announced as hunting has been very limited in recent years and there has been little interest in reviewing catch limits (ICES 2019).
Catches
The actual catches in recent years have been far below the TACs allowed. Reported Canadian catches have declined from a peak of around 355,000 in 2006 to 35,000 animals in 2015 (DFO 2016c). Although there was a slight increase to approximately 68,000 in 2016 and 81,000 in 2017, catches declined again in recent years to around 61,000 in 2019 and 32,000 in 2019. In 2019, the estimated catch was 8% of the TAC. The vast majority of harp seals taken in the Canadian commercial hunt are young of the year, accounting for over 97% of the reported catch since the late 1990s and up 10 100% in some years (ICES 2019).
The hunt of this stock also takes place in Arctic Canada and Greenland. Catches in the Canadian Arctic remain low, generally estimated at around 1,000 per year (ICES 2019). In Greenland, catches over the past decade have varied from 90,909 harp seals in 2010 to 48,593 in 2017, with an average of catch of 67,492 per year (ICES 2019).
Additional removals include by-catch, as well as estimates of animals killed, but not recovered (struck and lost). By-catch of harp seals in the Newfoundland lumpfish fisheries has been estimated from 1970-2003 (Sjare et al. 2005). However, since 2003 there have been signficant changes to this fishery and previous estimates have been revised. By-catch was low until the early 1990s due to limited effort in the fishery. This increased dramatically in the mid 1990s though and by-catch rose to over 45,000 seals per year (ICES 2019). Since 2010 though by-catch has remained low and in 2018 it was estimated to be 555 seals (ICES 2019). There is also a small number of harp seals from this stock caught by US fisheries.
Total removals from this stock have been under 250,000 animals each year since 2009 (DFO 2016c).
GREENLAND SEA STOCK
The Greenland Sea /Jan Mayen population was the first stock to be exploited commercially. The earliest recorded voyage for seals took place in 1720, and during the 18th and 19th centuries, ships from Germany, Holland, Denmark, Britain and later Norway took part in this hunt (Sergeant 1991).
At Jan Mayen, the catch began falling in the late 1850s, most likely due to over-hunting. From 1860 to 1900, an estimated total of 12.8 million seals were hunted from the West Ice (Kovacs 2015). The stock was reduced to a depleted state by the 1870s, and remained that way for around 100 years until effective management measures were introduced in the late 1970s.
Since the Jan Mayen seal hunt was carried out for 100 years before there are any reliable catch statistics, it is difficult to estimate the size of the initial stock. Dorofeev (1956 in Sergeant 1991) believed that it numbered no more than a million animals, and thought that numbers were kept low by high predation from polar bears, as well as by high wave action causing mortality of seal pups.
Although the population has been increasing, in 2013 it was thought to have not reached its historical population level (ICES 2013) and the rate of growth was slower than that seen in the Northwest Atlantic stock (Øigård et al. 2014). A significant drop in pup production has been documented from 2007 to 2018. This was from 110,530 pups estimated in 2007, to 89,590 in 2012 and down to 54,181 pups in 2018 (ICES 2019). In recent years, a decline in the extent and concentration of drift ice, particularly in the area north of Jan Mayan, which has traditionally been the main harp seal breeding area, has been observed (ICES 2019). Reductions in available ice are changing the harp seal breeding habitat in the Greenland Sea.
Catches
The annual quota for this stock has historically been around 20,000 animals. Between 2017-2019, the Total Allowable Catch for harp seals in the Greenland Sea was 26,000 animals older than one year (with 2 pups equal to a 1+ animal) (ICES 2016). Given the documented drop in pup production and a poor fit of the model to pup production estimates, the Joint ICES/NAFO/NAMMCO Working Group on Harp and Hooded Seals agreed to base catch options on the potential biological removal framework (PBR) instead of population modelling and recommended removals of no more than around 11,500 animals.
The total catch of harp seals in the Greenland Sea in recent years has been: 2000 animals (1934 of which were pups) in 2017; 2703 (including 1218 pups) in 2018, and; 5813 (including 2168 pups) in 2019 (ICES 2019). Because the hunt from this stock is low and well below that recommended as being sustainable, the population should continue to increase. However, the documented decline in pup production may indicate that there are currently sub-optimal breeding conditions available or that the population could be approaching its current carrying capacity (Øigård et al. 2014).
No Russian vessels have hunted in this area since 1994 and only 1 Norwegian vessel took part in the hunt in 2017 and 2018, while 2 Norwegian vessels were involved in 2019 (ICES 2019). Up until 2014, seal hunts were subsidised by the Norwegian government. These were removed in 2015 and reinstated at a considerably lower level in 2016. This level of support was maintained in 2017-2019. The reductions in catch rates are a result from changes in hunt effort rather than an indication of changes in stock abundance or availability.
BARENTS SEA/WHITE SEA STOCK
The population size for the Barents Sea/White Sea stock is estimated to have been around 6 million seals in 1875 (though with a range from 3-7 million seals, depending on the model used), when large-scale exploitation by Norwegian and Russian hunters started (Skaug et al. 2007). The Barents Sea/White Sea population became over-exploited during the first half of the twentieth century due to this hunting pressure. A maximum catch of more than 400,000 seals was taken in 1925 (Sergeant 1991). There was an increase in population size during the period 1940–1945 resulting from the temporary halt in hunting during World War II (Skaug et al. 2007). However, after hunting resumed, the population was reduced from approximately 1.5 million seals in the early 1950s to 500,000 in the early 1960s – an historical low (Potelov I 2003, Johnston et al. 2012).
Catches were reduced and regulated by the USSR in 1965, with a strict quota catch taken by the USSR, and the population has been increasing since then (Skaug et al. 2007). The population recovered to approximately 800,000 by 1978 (Johnston et al. 2012) and was estimated to have reached about 1 million by 1980, with a continued increase of about 5% annually during the 1980s (Sergeant 1991). The modeled total population in 2013 was estimated to be about 83% of the historical maximum population size observed/estimated (ICES 2013).
Reductions in pup production and a decline in body condition have been documented in this stock, thought to be due to warmer water and a loss of sea ice cover due to environmental change (Kovacs 2015, Johnston et al. 2012). Due to the sharp decline in pup production observed after 2003, ICES (2016) recommended that removals be restricted to the estimated sustainable equilibrium level. For 2017-2019, this was around 10,000 1+ animals (with 2 pups being equal to a 1+ animal). The Joint Norwegian-Russian Fisheries Commission followed this proposal for the total allowable catch and allocated 7000 seals to Norway and approximately 3000 to Russia (ICES 2019).
Catches
A ban implemented on all pup catches prevented the Russian hunt in the White Sea between 2009-2013. Although this bas was removed before the 2014 season, the availability of ice was too restricted to permit sealing, resulting in no commercial Russian harp seal catches in the White Sea from 2014-2019.
No Norwegian commercial hunting vessels operated in the southeastern Barents Sea in 2017 (although one animal was taken for scientific purposes north of Svalbard). One Norwegian vessel hunted in the area in 2018 and 2019 and the total catch was 2241 animals (including 21 pups) in 2018 and 602 animals (including 34 pups) in 2019 (ICES 2019).
Any by-catch of harp seals along the Norwegian coast is assumed to come from the Barents Sea/White Sea population. However no reported data is available on this since 1991 (ICES 2019).
MANAGEMENT
The individual stocks fall under management bodies within Canada, Greenland, Norway, and Russia. These countries are advised by a joint scientific working group (WGHARP) between NAMMCO, the International Council for the Exploration of the Seas (ICES), and the Northwest Atlantic Fisheries Organization (NAFO).
NORTHWEST ATLANTIC STOCK
Management measures for this stock were first introduced in the 1960s, following an observed reduction in the population. In the mid 1960s, adult females were protected on the breeding grounds, and Norway was excluded from sealing in Canadian waters (Kovacs 2015). In 1972, the Gulf of St. Lawrence was closed to seal hunting vessels and a quota was introduced (Sergeant 1991).
Atlantic Seal Management Strategy
Northwest Atlantic harp seals are currently managed under the Atlantic Seal Management Strategy implemented by the Canadian Department of Fisheries and Oceans. They are considered to be a data-rich population and have been managed for the last decade under a “precautionary approach” with the objective of maintaining an 80% probability that the population remains above a target reference level (N70) which is defined to be 70% of the maximum estimated population size (DFO 2016c).
Two other reference levels are identified under this strategy: a precautionary reference point and the limit reference point. The precautionary point is set at N50, or 50% of the maximum estimated population size. If the population was to drop below this level, then significant conservation measures would be implemented. If the population reached the limit reference point, N30 or 30% of the maximum population, then all removals would be stopped (DFO 2016b).
Seasons, areas and total allowable catch
The seasons and hunting areas for harp seal hunt are set out in the Marine Mammal Regulations (MMR) under Canada’s Fishery Act. The season for the commercial hunt of harp seals is established annually in consultation with sealing fleets and set out in Variation Orders pursuant to the MMR, taking into account environmental and biological conditions. It can be adjusted as needed by Variation Orders to the Regulations to accommodate changing circumstances (DFO 2016b).
Under the Atlantic Seal Management Strategy, a multiyear Total Allowable Catch (TAC) is established for three consecutive seasons. The Minister of Fisheries and Oceans can change this and set an annual TAC if needed to account for new information that might be obtained on the status of the population, changing environmental conditions, or changes in catch levels in Arctic Canada and Greenland.
The overall TAC of harp seals is subdivided into commercial sealing allocations applicable to different areas and fleet sectors, a personal use allocation for all areas, a subsistence allocation for northern communities, and a developmental allocation for special projects that might encourage new applications in the seal industry (DFO 2016b).
GREENLAND SEA STOCK
Management measures for this stock were implemented as early as 1876, after it became evident that catch levels in the 1870s were higher than the stock could sustain. The first measures imposed were mainly designed to protect adult females (Øigård et al. 2014). The stock remained in a depleted state for about 100 years, until quotas on the overall hunt were imposed in 1971 (Øigård et al. 2014).
Quotas for sealing in the northeast Atlantic (Greenland Sea stock) are currently based on recommendations made by the Joint ICES/NAFO/NAMMCO scientific Working Group (WGHARP). Canada, Norway and Denmark-Greenland are voting members of NAFO.
Norway is responsible for managing stocks in the Greenland Sea near Jan Mayen Island, and banned the killing of whitecoats from this stock in 1989 (Kovacs 2015). Harp seals are protected in Svalbard, with no hunting allowed.
BARENTS SEA/WHITE SEA STOCK
The Barents Sea/White Sea population was over-exploited during the first half of the twentieth century. Heavy hunting of seals by ships began in the 1920s and 1930s, first by Norway and then by the Soviet Union. Depletion of the population led to catch regulations, with a strict quota on the hunt being implemented by the USSR in 1965 (Potelov et al. 2003, Sergeant 1991).
Russia is currently responsible for managing Harp Seals in the southeast part of the Barents Sea, and banned the killing of whitecoats there in 2009 (Kovacs 2015).
Total Allowable Catch advice for the Barents Sea/White Sea is given by the WGHARP and quotas are currently allocated by the Joint Norwegian-Russian Fisheries Commission (ICES 2019).
HUNTING AND UTILISATION
All three populations of harp seals have been hunted for thousands of years by the Indigenous Peoples of the Atlantic Arctic, as well as by coastal Northern Europeans. Commercial exploitation of these stocks has a history of some hundreds of years. Currently, harp seals are taken in the Canadian and Norwegian commercial hunts, and in the Greenland and Canadian Arctic subsistence hunts.
NORTHWEST ATLANTIC STOCK
Historically
Although harp seals have been hunted commercially in Atlantic Canada since the 1700s, the highest level of catches occurred between 1818 and 1862, when around 500,000 seals were hunted annually with maximum yearly takes of up to 740,000 seals (Kovacs 2015). Following this period, catches declined significantly, reaching a minimum of 15,300 per year during World War II (Stenson 2014).
After the war, exploitation of this stock by Canada and Norway increased. Canadian commercial catches during this period averaged around 288,000 harp seals annually. This led to a reduction in the population and the introduction of management measures. In the mid 1960s, adult females became protected on the breeding grounds, and Norway was excluded from sealing in Canadian waters (Kovacs 2015). In 1972, the Gulf of St. Lawrence was closed to seal hunting ships and a quota was introduced (Sergeant 1991).
In more recent decades, the catch has varied. Between 1972 and the end of the large vessel hunt in 1982, an average of 165,000 seals was taken each year. Catches decreased after 1982, after the European Economic Community imposed an import ban on all whitecoat products, and has remained low, averaging around 52,000, until 1995. In 1987, Canada banned the killing of whitecoats and the focus of the hunt switched to “beaters” (post-weaning to 13 months of age).
Recent years
Annual catches increased after 1995 to an average of 272,600 between 1996 and 2006. However, after more than a decade of high catches, levels have remained below 100,000 since 2009, with an average of around 63,000 animals (ICES 2019). Catches have declined due to ice conditions and poor markets and reached a low of 32,038 in 2019 (ICES 2019).
The total value of the seal hunt has decreased since 2006, when it was valued at over $34 million and sealers received over $100 per pelt. Significant drops in both the quantity and value of hunted pelts, as well as large fluctuations in pelt prices, mainly accounted for this trend. The value of the catch remained low in 2010 ($1.3 million) and the price for a pelt was approximately $20 (DFO 2016b).
Harp seals are also taken in subsistence hunts in Labrador, Newfoundland, northern Quebec and Nunavut, as well as in Greenland. Catches in the Canadian Arctic are not well documented but appear to be low with an estimated fewer than 1,000 harp seals taken yearly (ICES 2019). The Greenland hunt has varied greatly in recent years with reported catches ranging from a high of around 90,000 in 2010 to as low as 48,000 in 2018 (ICES 2019). Subsistence hunts in both Canada and Greenland are not limited by quotas.
NORTHEAST ATLANTIC / GREENLAND SEA STOCK
This was the first stock to be exploited commercially. The earliest recorded voyage for seals took place in 1720, and during the 18th and 19th centuries ships from Germany, Holland, Denmark, Britain and later Norway took part in this hunt (Sergeant 1991).
Catches peaked in the late 1800s with annual catches of from 50,000 to 120,000 seals (Øigård et. al 2014). In the 20th century, catches increased to an average of more than 100,000 per year, reaching a maximum in the 1920s and 1930s when the catches were 200,000-300,000 per year. In 1989, Norway banned the killing of whitecoats and catches in the last few decades have been small compared to historical numbers (Kovacs 2015).
At Jan Mayen, catches began falling in the late 1850s, likely due to over-exploitation. Between 1860 and 1900, an estimated 12.8 million harp seals were hunted from the West Ice / Greenland Sea stock (Kovacs 2015). It was evident in the 1870s that the catch levels were higher than the stock could sustain, and some regulatory measures (mainly designed to protect adult females) were taken in 1876 (Øigård et al. 2014). In the first decades of the 20th century Norway was the only hunting nation in the area, and the harp seal catches varied between 10,000 and 20,000 animals. The hunt increased to around 40,000 seals annually in the 1930s (Sergeant 1991). After a 5-year pause in the sealing operations during World War II, total annual catches quickly rose to a post-war maximum of about 70,000 in 1948, but then followed a decreasing trend until quotas were imposed in 1971.
Recent years
Since 1983, when Europe banned the importation of products from whitecoat pups, catches have decreased to under 10,000 animals in most years. No Russian vessels have hunted in the area since 1994. In 2015, Norway ceased subsidizing the sealing industry, and although subsidies were reintroduced in 2016, this was at a considerably lower level than previously. The hunt effort in the area has remained low since this that time and the total catch of the Greenland Sea population in in 2018 was 2703 animals, and 5813 animals in 2019 (ICES 2019).
WHITE SEA / BARENTS SEA STOCK
This stock has been subjected to extensive Norwegian and Russian commercial hunting since the 1870s. Heavy hunting of seals by both of these countries occurred in the 1920s and 1930s, and in 1925 a maximum catch of over 400,000 seals was taken.
Harp seals were hunted both on the ice and from shore. Sdobnikov (1933, in Sergeant 1991) described a spring net fishery for harp seals, which took place at that time on the Murman coast. Seals were also hunted from shore on both coasts and around the Kanin Peninsula, and later in the season from boats (Sergeant 1991).
This extensive hunting, particularly the practice of Soviet ships of taking large numbers of whelping females, severely depleted the population and may have reduced it to an historical low (Skaug et al. 2007, Potelov et al. 2003). Catches were reduced and regulated by the USSR in 1965, with a strict quota placed on the catch taken (Sergeant 1991).
Russia banned the killing of whitecoats in 2009 and the White Sea hunt has been almost negligible since that time, with annual takes of between 0 and 200 seals (Kovacs 2015). A ban implemented on all pup catches prevented Russian hunt in the White Sea during the period 2009–2013. This ban was removed before the 2014 season. However, the availability of ice was too restricted to permit sealing, resulting in no commercial Russian harp seal catches in the White Sea in 2014–2019 (ICES 2019).
Norwegian catches of this population during summer when they are in the Barents Sea have also been very small in recent years, with no hunt at all in 2013 (ICES 2013). No Norwegian vessels operated in the southeastern Barents Sea in 2017, while one Norwegian vessel hunted in the area in both 2018 and 2019. Total catches of harp seals were 1 in 2017 (taken north of Svalbard for scientific purposes), 2241 (including 21 pups) in 2018, and 602 (including 34 pups) in 2019 (ICES 2019).
STRUCK AND LOST
“Struck and lost” occurs when an animal is hit (struck) by a weapon, such as a rifle bullet or harpoon, but is not retrieved by the hunter. The injured animal may survive or die, depending on the severity of the injury (NAMMCO 2006). Even if animals survive, their reproductive contribution to the population may be diminished. Struck and lost is a source of mortality, just as is direct catch, and should be incorporated into estimates of removals and population models. However struck and lost is very difficult to estimate, as it generally requires intensive monitoring of hunting activities by an independent observer (NAMMCO 2006).
Rates
Struck and loss rates vary greatly depending on the age of the seal taken, the hunter’s experience, the type of hunt, and the weather conditions at the time of the hunt. For young of the year in the Canadian commercial hunt, it is assumed that 95% of the seals are recovered (Stenson 2014). For older animals, Sjare and Stenson (2002) estimated loss rates to vary between 0 and 21.6 % on the ice and 5 to 50% when taken in the water. Struck and lost rates are very low for seals shot on the ice in the commercial hunt, ranging from 0 to 2% of struck animals. Loss rates are higher for those struck in the water, ranging up to 10% in some hunts (NAMMCO 2004).
Struck and lost rates in the Greenlandic open water hunt for harp seals has been a matter of some controversy. Current population models assume a loss rate of 50%, which is considered too high by most Greenlandic hunters (NAMMCO 2006, Stenson 2014). A questionnaire survey suggested that the average loss rate for Greenlandic hunters was about 22% (NAMMCO 2006), and confirmed that loss rates were highest in May and June, when seals have a lower blubber content, are less buoyant and sink before they can be retrieved.
In the Norwegian harp seal hunt, shooting of animals in the water is prohibited (if not injured). Hence, struck and lost rates are low for adult seals, and weaned pups float when they are dead.
Catches in NAMMCO member countries since 1992
| Country | Species (common name) | Species (scientific name) | Year or Season | Area or Stock | Catch Total | Quota (if applicable) |
|---|---|---|---|---|---|---|
| Greenland | Harp seal | Pagophilus groenlandicus | 2023 | Northwest Atlantic | 24742 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2023 | Greenland Sea | 1081 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2023 | Total | 25823 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2022 | Greenland Sea | 929 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2022 | Northwest Atlantic | 28751 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2022 | Total | 29680 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2021 | Northwest Atlantic | 29904 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2021 | Greenland Sea | 773 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2021 | Total | 30667 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2020 | Greenland Sea | 1403 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2020 | Northwest Atlantic | 48759 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2020 | Total | 50162 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2019 | Greenland Sea | 2495 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2019 | Northwest Atlantic | 42442 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2019 | Total | 44937 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2018 | East | 1754 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2018 | West | 45589 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2018 | Total | 47343 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2017 | East | 1103 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2017 | West | 47854 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2017 | Total | 48957 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2016 | East | 1359 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2016 | West | 56328 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2016 | Total | 57687 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2015 | East | 865 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2015 | West | 62076 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2015 | Total | 62941 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2014 | East | 940 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2014 | West | 63055 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2014 | Total | 63995 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2013 | East | 1280 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2013 | West | 80822 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2013 | Total | 82102 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2012 | East | 973.5 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2012 | West | 59882.5 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2012 | Total | 60856 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2011 | East | 896 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2011 | West | 73481 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2011 | Total | 74377 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2010 | East | 991 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2010 | West | 89755 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2010 | Total | 90746 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2009 | East | 1533 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2009 | West | 71812 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2009 | Total | 73345 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2008 | East | 3440 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2008 | West | 78732 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2008 | Total | 82172 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2007 | East | 1430 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2007 | West | 82272 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2007 | Total | 83702 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2006 | East | 1039 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2006 | West | 92210 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2006 | Total | 93249 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2005 | East | 1426 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2005 | West | 91696 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2005 | Total | 93122 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2004 | East | 1518 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2004 | West | 70441 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2004 | Total | 71959 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2003 | East | 1447 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2003 | West | 66759 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2003 | Total | 68206 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2002 | East | 822 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2002 | West | 50302 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2002 | Total | 51124 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2001 | East | 1208 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2001 | West | 78068 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2001 | Total | 79276 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2000 | East | 1289 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2000 | West | 98589 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 2000 | Total | 99878 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 1999 | Total | 95023 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 1998 | Total | 82491 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 1997 | Total | 69663 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 1996 | Total | 74758 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 1995 | Total | 57812 | No quota |
| Greenland | Harp seal | Pagophilus groenlandicus | 1994 | Total | 55576 | No quota |
| Iceland | Harp seal | Pagophilus groenlandicus | 2023 | Iceland | 0 | |
| Iceland | Harp seal | Pagophilus groenlandicus | 2022 | Iceland | 0 | |
| Iceland | Harp seal | Pagophilus groenlandicus | 2021 | Iceland | 0 | |
| Iceland | Harp seal | Pagophilus groenlandicus | 2020 | Iceland | 0 | |
| Iceland | Harp seal | Pagophilus groenlandicus | 2019 | Iceland | 0 | |
| Iceland | Harp seal | Pagophilus groenlandicus | 2018 | Iceland | 0 | |
| Iceland | Harp seal | Pagophilus groenlandicus | 2011-2017 | Iceland | N/A | |
| Iceland | Harp seal | Pagophilus groenlandicus | 2010 | Iceland | 35 | |
| Iceland | Harp seal | Pagophilus groenlandicus | 2005-2009 | Iceland | N/A | |
| Iceland | Harp seal | Pagophilus groenlandicus | 2004 | Iceland | 5 | |
| Iceland | Harp seal | Pagophilus groenlandicus | 2003 | Iceland | 1 | |
| Iceland | Harp seal | Pagophilus groenlandicus | 2002 | Iceland | 1 (+124 by Norwegians) | |
| Iceland | Harp seal | Pagophilus groenlandicus | 1992-2001 | Iceland | N/A | |
| Norway | Harp seal | Pagophilus groenlandicus | 2023 | Norway coast | No quota | |
| Norway | Harp seal | Pagophilus groenlandicus | 2023 | Greenland Sea | 1881 | 11548 |
| Norway | Harp seal | Pagophilus groenlandicus | 2023 | White Sea/Barents Sea | 7000 | |
| Norway | Harp seal | Pagophilus groenlandicus | 2023 | Total | 1881 | |
| Norway | Harp seal | Pagophilus groenlandicus | 2022 | Greenland Sea | 1421 | 11548 |
| Norway | Harp seal | Pagophilus groenlandicus | 2022 | White Sea/Barents Sea | 0 | 7000 |
| Norway | Harp seal | Pagophilus groenlandicus | 2022 | Total | 1423 | |
| Norway | Harp seal | Pagophilus groenlandicus | 2021 | Norway coast | 1 | No quota |
| Norway | Harp seal | Pagophilus groenlandicus | 2021 | Greenland Sea | 10 | 11548 |
| Norway | Harp seal | Pagophilus groenlandicus | 2021 | White Sea/Barents Sea | 5084 | 7000 |
| Norway | Harp seal | Pagophilus groenlandicus | 2021 | Total | 5095 | N/A |
| Norway | Harp seal | Pagophilus groenlandicus | 2020 | White Sea/Barents Sea | 0 | 7000 |
| Norway | Harp seal | Pagophilus groenlandicus | 2020 | West Ice | 10284 | 11548 |
| Norway | Harp seal | Pagophilus groenlandicus | 2020 | Norway coast | 1 | No quota |
| Norway | Harp seal | Pagophilus groenlandicus | 2020 | Total | 10285 | |
| Norway | Harp seal | Pagophilus groenlandicus | 2019 | East Ice | 602 | 10090 (+1) |
| Norway | Harp seal | Pagophilus groenlandicus | 2019 | West Ice | 5813 | 26000 (+1) |
| Norway | Harp seal | Pagophilus groenlandicus | 2019 | Nowegian Coast | 0 | No quota |
| Norway | Harp seal | Pagophilus groenlandicus | 2019 | Total | 6415 | |
| Norway | Harp seal | Pagophilus groenlandicus | 2018 | East Ice | 2241 | 10090 (+1) |
| Norway | Harp seal | Pagophilus groenlandicus | 2018 | West Ice | 2703 | 26000 (+1) |
| Norway | Harp seal | Pagophilus groenlandicus | 2018 | Total | 4944 | |
| Norway | Harp seal | Pagophilus groenlandicus | 2017 | East Ice | 1 | |
| Norway | Harp seal | Pagophilus groenlandicus | 2017 | West Ice | 2000 | 26000 |
| Norway | Harp seal | Pagophilus groenlandicus | 2017 | Total | 2001 | |
| Norway | Harp seal | Pagophilus groenlandicus | 2016 | East Ice | 28 | |
| Norway | Harp seal | Pagophilus groenlandicus | 2016 | West Ice | 1442 | |
| Norway | Harp seal | Pagophilus groenlandicus | 2016 | Finnmark | 1 | |
| Norway | Harp seal | Pagophilus groenlandicus | 2016 | Total | 1471 | |
| Norway | Harp seal | Pagophilus groenlandicus | 2015 | East Ice | 0 | |
| Norway | Harp seal | Pagophilus groenlandicus | 2015 | West Ice | 2237 | |
| Norway | Harp seal | Pagophilus groenlandicus | 2015 | Total | 2237 | |
| Norway | Harp seal | Pagophilus groenlandicus | 2014 | East Ice | 0 | |
| Norway | Harp seal | Pagophilus groenlandicus | 2014 | West Ice | 11986 | |
| Norway | Harp seal | Pagophilus groenlandicus | 2014 | Total | 11986 | |
| Norway | Harp seal | Pagophilus groenlandicus | 2013 | East Ice | 0 | |
| Norway | Harp seal | Pagophilus groenlandicus | 2013 | West Ice | 15939 | |
| Norway | Harp seal | Pagophilus groenlandicus | 2013 | Total | 15939 | |
| Norway | Harp seal | Pagophilus groenlandicus | 2012 | East Ice | 0 | |
| Norway | Harp seal | Pagophilus groenlandicus | 2012 | West Ice | 5593 | |
| Norway | Harp seal | Pagophilus groenlandicus | 2012 | Finnmark | 4 | |
| Norway | Harp seal | Pagophilus groenlandicus | 2012 | Total | 5597 | |
| Norway | Harp seal | Pagophilus groenlandicus | 2011 | East Ice | 200 | |
| Norway | Harp seal | Pagophilus groenlandicus | 2011 | West Ice | 10132 | |
| Norway | Harp seal | Pagophilus groenlandicus | 2011 | Total | 10332 | |
| Norway | Harp seal | Pagophilus groenlandicus | 2010 | East Ice | 125 | |
| Norway | Harp seal | Pagophilus groenlandicus | 2010 | West Ice | 4672 | |
| Norway | Harp seal | Pagophilus groenlandicus | 2010 | Total | 4797 | |
| Norway | Harp seal | Pagophilus groenlandicus | 2009 | East Ice | 0 | |
| Norway | Harp seal | Pagophilus groenlandicus | 2009 | West Ice | 8035 | |
| Norway | Harp seal | Pagophilus groenlandicus | 2009 | Total | 8035 | |
| Norway | Harp seal | Pagophilus groenlandicus | 2008 | East Ice | 0 | |
| Norway | Harp seal | Pagophilus groenlandicus | 2008 | West Ice | 1263 | |
| Norway | Harp seal | Pagophilus groenlandicus | 2008 | Total | 1263 | |
| Norway | Harp seal | Pagophilus groenlandicus | 2007 | East Ice | 6153 | |
| Norway | Harp seal | Pagophilus groenlandicus | 2007 | West Ice | 7828 | |
| Norway | Harp seal | Pagophilus groenlandicus | 2007 | Total | 13981 | |
| Norway | Harp seal | Pagophilus groenlandicus | 2006 | East Ice | 10086 | |
| Norway | Harp seal | Pagophilus groenlandicus | 2006 | West Ice | 3304 | |
| Norway | Harp seal | Pagophilus groenlandicus | 2006 | Finnmark | 4 | |
| Norway | Harp seal | Pagophilus groenlandicus | 2006 | Total | 13394 | |
| Norway | Harp seal | Pagophilus groenlandicus | 2005 | East Ice | 10566 | |
| Norway | Harp seal | Pagophilus groenlandicus | 2005 | West Ice | 7205 | |
| Norway | Harp seal | Pagophilus groenlandicus | 2005 | Finnmark | 8 | |
| Norway | Harp seal | Pagophilus groenlandicus | 2005 | Total | 17779 | |
| Norway | Harp seal | Pagophilus groenlandicus | 2004 | East Ice | 33 | |
| Norway | Harp seal | Pagophilus groenlandicus | 2004 | West Ice | 9895 | |
| Norway | Harp seal | Pagophilus groenlandicus | 2004 | Total | 9928 | |
| Norway | Harp seal | Pagophilus groenlandicus | 2003 | East Ice | 5298 | |
| Norway | Harp seal | Pagophilus groenlandicus | 2003 | West Ice | 2277 | |
| Norway | Harp seal | Pagophilus groenlandicus | 2003 | Total | 7575 | |
| Norway | Harp seal | Pagophilus groenlandicus | 2002 | East Ice | 2348 | |
| Norway | Harp seal | Pagophilus groenlandicus | 2002 | West Ice | 1232 | |
| Norway | Harp seal | Pagophilus groenlandicus | 2002 | Total | 3580 | |
| Norway | Harp seal | Pagophilus groenlandicus | 2001 | East Ice | 5200 | |
| Norway | Harp seal | Pagophilus groenlandicus | 2001 | West Ice | 2992 | |
| Norway | Harp seal | Pagophilus groenlandicus | 2001 | Total | 8192 | |
| Norway | Harp seal | Pagophilus groenlandicus | 2000 | East Ice | 6357 | |
| Norway | Harp seal | Pagophilus groenlandicus | 2000 | West Ice | 12343 | |
| Norway | Harp seal | Pagophilus groenlandicus | 2000 | Total | 18700 | |
| Norway | Harp seal | Pagophilus groenlandicus | 1999 | East Ice | 1150 | |
| Norway | Harp seal | Pagophilus groenlandicus | 1999 | West Ice | 803 | |
| Norway | Harp seal | Pagophilus groenlandicus | 1999 | Total | 1953 | |
| Norway | Harp seal | Pagophilus groenlandicus | 1998 | East Ice | 832 | 5000 |
| Norway | Harp seal | Pagophilus groenlandicus | 1998 | West Ice | 1884 | 13100 |
| Norway | Harp seal | Pagophilus groenlandicus | 1998 | Total | 2716 | 18100 |
| Norway | Harp seal | Pagophilus groenlandicus | 1997 | East Ice | 5019 | 5000 |
| Norway | Harp seal | Pagophilus groenlandicus | 1997 | West Ice | 2161 | 13100 |
| Norway | Harp seal | Pagophilus groenlandicus | 1997 | Total | 7180 | 18100 |
| Norway | Harp seal | Pagophilus groenlandicus | 1996 | East Ice | 9521 | 9500 |
| Norway | Harp seal | Pagophilus groenlandicus | 1996 | West Ice | 6427 | 10600 |
| Norway | Harp seal | Pagophilus groenlandicus | 1996 | Total | 15948 | 20100 |
| Norway | Harp seal | Pagophilus groenlandicus | 1995 | East Ice | 6842 | 8750 |
| Norway | Harp seal | Pagophilus groenlandicus | 1995 | West Ice | 8206 | 10140 |
| Norway | Harp seal | Pagophilus groenlandicus | 1995 | Total | 15048 | 18890 |
| Norway | Harp seal | Pagophilus groenlandicus | 1994 | East Ice | 9500 | |
| Norway | Harp seal | Pagophilus groenlandicus | 1994 | West Ice | 8121 | |
| Norway | Harp seal | Pagophilus groenlandicus | 1994 | Total | 17621 | |
| Norway | Harp seal | Pagophilus groenlandicus | 1993 | East Ice | 8758 | 9500 |
| Norway | Harp seal | Pagophilus groenlandicus | 1993 | West Ice | 3520 | 8400 |
| Norway | Harp seal | Pagophilus groenlandicus | 1993 | Total | 12278 | 17900 |
| Norway | Harp seal | Pagophilus groenlandicus | 1992 | East Ice | 5623 | |
| Norway | Harp seal | Pagophilus groenlandicus | 1992 | West Ice | 7750 | |
| Norway | Harp seal | Pagophilus groenlandicus | 1992 | Total | 13373 | |
This database of reported catches is searchable, meaning you can filter the information by for instance country, species or area. It is also possible to sort it by the different columns, in ascending or descending order, by clicking the column you want to sort by and the associated arrows for the order. By default, 30 entries are shown, but this can be changed in the drop-down menu, where you can decide to show up to 100 entries per page.
Carry-over from previous years are included in the quota numbers, where applicable.
You can find the full catch database with all species here.
For any questions regarding the catch database, please contact the Secretariat at nammco-sec@nammco.no.
EU SEALSKIN BAN AND THE INUIT EXEMPTION
In 1983, the European Economic Community imposed an import ban on all whitecoat products (Kovacs 2015). The ban, which was initially for 2 years, has been extended since then and was expanded in 2009 to include juvenile (beater) pelts (Hammill et al. 2015).
Exceptions to the ban include products resulting from Inuit/Indigenous hunts; products for the sole purpose of sustainable management of marine resources; and products for the personal use of travelers to the EU. As well, the ban does not apply to seal products trans-shipped through the EU to non-EU destinations (DFO 2016b).
In order for seal products to be exempted from the ban and placed on the market in the EU the Inuit or Indigenous hunt must meet certain conditions. The hunt must:
- be traditionally conducted by the community
- contribute to the community’s subsistence in order to provide food and income and not be primarily conducted for commercial reasons
- pay due care to animal welfare, while taking account of the community’s way of life and the subsistence purpose of the hunt (EUR-Lex 2016).
BY-CATCH
Harp seals are caught in commercial fishing gear (particularly bottom set gillnets) in many parts of their range. Estimates of harp seal by-catch in the Newfoundland lumpfish fishery increased from fewer than 1,000 in the early 1970s to a peak of 46,400 in 1994 (Stenson 2014). Since then, estimates of the by-catch from this fishery have declined, reaching approximately 5,000 by 2003. Currently, lumpfish catches are generally low, and the actual by-catch levels are unknown. Low numbers of harp seals are also caught in commercial fisheries based in the northeastern United States (Stenson 2014).
CLIMATE CHANGE
Like other Arctic marine mammals, harp seals can be impacted by climate change both directly through a loss of ice habitat and indirectly through the way climate change can alter prey assemblages and foraging ecology. Recent research has documented how the three populations of harp seals in the North Atlantic (White Sea/Barents Sea, Greenland Sea, and Northwest Atlantic) are experiencing varying degrees and types of environmental change and how this variation is reflected in different impacts on abundance, body condition and reproduction across the three populations (Stenson et al. 2020).
Sea ice
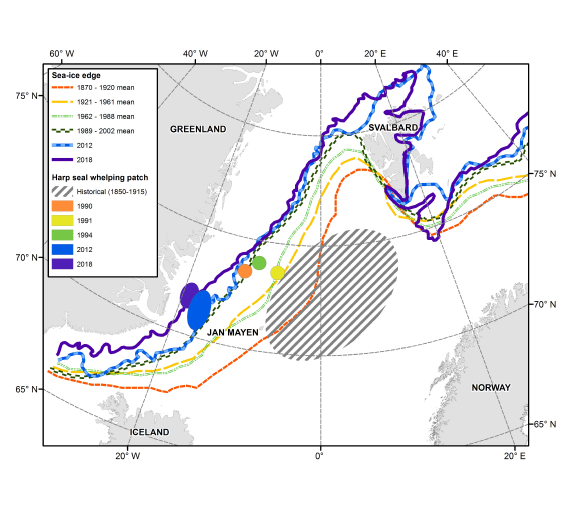
The location of the sea ice edge and Greenland Sea harp seal whelping concentrations (from Stenson et al. 2020).
Because the harp seal is so dependent on sea ice for pupping, moulting and resting, they will be particularly affected by any changes that might occur in their sea ice habitats. Sea water temperatures have varied historically, but over the past four decades there has been a warming trend in the water temperatures throughout the harp seal’s range. Arctic sea ice conditions have also changed dramatically over the last 25 years.There has been a decline in sea ice extent and coverage associated with this warming trend since the beginning of the satellite record of ice conditions (1979), particularly in the southern wintering and pupping area of the Northwest Atlantic stock (Johnston et al. 2012). Since 2000, the frequency of light ice years (measured primarily as ice cover) in these areas has increased and the duration of the ice season decreased (Bajzak et al. 2011, DFO 2010), with 4 of the past 7 years being among the lowest ice cover ever recorded (Stenson et al. 2016). A decline in the extent and concentration of sea ice available to the Greenland Sea stock in traditional pupping areas has also been seen in recent years (ICES 2019). In recent years, the ice edge has also shifted westward. This has moved the location of pupping closer to the Greenland coast, which makes harp seals more vulnerable to predation from polar bears and killer whales occupying coastal waters (Stenson et al. 2020).
Poor ice conditions (extent, coverage, and/or thickness) have been shown to directly affect harp seals on their whelping grounds. Stenson and Hammill (2014) found that pup mortality was extremely high in years with reduced ice coverage or thickness. Factors contributing to this increased mortality include interrupted nursing, starvation, cold stress and crushing by shifting floes if pups are prematurely forced into the water by the rapid melting and break-up of ice (Hammill and Stenson 2014, Johnston et al. 2012, Bajzak et al. 2011).
Prey availability
In the Northwest Atlantic, changes in winter prey availability, particularly capelin, due to warmer waters, results in females with poor body condition who may not be able to carry their pups to full term (Stenson et al. 2016, Hammill et al. 2015). Reductions in pup production and declines in body condition have also been documented in the Northeast Atlantic and Barents Sea/White Sea breeding groups, and are also thought to be due to environmental change and reduced prey availability linked to warmer water and less sea ice (Kovacs 2015, Johnston et al. 2012, Stenson et al. 2020).
Adaptations
Harp seals might adapt to these environmental changes by changing the timing of birth or by finding areas with more suitable ice. In the Gulf herd, there is some evidence for a shift in the timing of births, with females giving birth earlier in the season (Hammill and Stenson 2014). Harp seals have not shown similar signs of adjusting the timing of births at the Front, but in years of very little ice cover some have responded by moving further north to more suitable ice as was seen in 2010 (Stenson and Hammill 2012). Harp seals whelping off East Greenland (the West Ice) have also been seen to be moving further north to find suitable ice (Johnston et al. 2012).
SHIPPING / OIL SPILLS
Another potential threat to harp seals brought about by climate change is the increasing ship traffic in the Arctic due to reduced ice cover and more open water. The dramatic decline in Arctic sea ice over the past few years has raised the possibility of regular cargo traffic being diverted from current shipping routes. Shipping traffic in the Arctic is also predicted to lead to increases in other activities, including resource extraction and tourism (Corbett et al. 2010). Ships could affect harp seal habitat through their emissions or an accidental fuel spill. They could also affect the ecosystem by introducing invasive species, or disrupt the seals themselves with their movements and noise.
Oil spills can affect both harp seal habitat and the seals themselves. An accidental fuel spill in 1969 from a ruptured tank on the shores of New Brunswick, Canada led to some 10,000 to 15,000 seals being heavily coated with oil and resulted in a high mortality of pups (Kovacs 2015). Oil development in the Barents Sea is ongoing and could pose a threat to harp seals in the Barents Sea/White Sea and Greenland Sea stocks.
CONTAMINANTS
Harp seals have been found to carry significant loads of contaminants including heavy metals, DDT and PCBs , although it is not known whether or not these affect harp seal health (Kovacs 2015).
FISHING
Reduced availability of potential prey due to fishing (especially of polar cod, capelin and herring) is a threat to harp seals. When their preferred prey is not available, seals migrate out of their normal range. Several harp seal “invasions” took place in the late 1980s along the north coast of Norway and resulted in large numbers of seals killed as by-catch in fishing nets (Kovacs 2015).
NORWAY
In Norway, research on harp seals is conducted through the Norwegian Polar Institute and the Norwegian Institute of Marine Research. Some of the research is conducted under the MOSJ programme -Environmental Monitoring of Jan Mayen and Svalbard.
Surveys
The last aerial survey of the Greenland Sea pupping areas was carried out in 2018, with photographs taken from fixed-wing aircraft in order to get estimates of pup production and a helicopter flying reconnaissance flights and monitoring the distribution of seal patches and age-staging the pups (ICES 2019). Similar surveys were flown in 2012, 2007, 2002 and 1991.
The survey methods used are under continuous evaluation and development. The use of satellite imagery for detection of new whelping areas is being considered, as is the use of drones to replace the fixed-wing aircraft for the photographic surveys. Inspecting the aerial photos and counting the pups takes much time and effort, and research is ongoing to determine the extent to which the process can be automated. Methods for software-based detection using artificial intelligence (deep learning) is currently being developed through a collaboration between the Norwegian Computing Centre and Institute of Marine Research, Norway and Fisheries and Oceans, Canada. Early results indicate the potential for automatic detection of seal pups, however, work remains ongoing to improve the level of false positives since there has been variation in effectiveness when used for the Greenland Sea and the Northwest Atlantic stocks. The development of a semi-automatic approach where the reader validates the automatic detections appears to be feasible though.
Biology, Ecology & Contaminants
Data for assessment of biological parameters (growth, condition, age at maturity, fertility) were collected from 176 harp seal females during Norwegian commercial sealing in the West Ice in 2019 and analyses of these are in progress (National Progress Report Norway 2020).
In addition, in 2019 8 adult and 6 new-born harp seals from the Greenland Sea stock were culled by researchers from the Norwegian Institute of Marine Research for use in various collaborative scientific projects. Samples were, for example, used in studies on the patterns of entry and accumulation of mercury (Hg), including how feeding behaviour and distribution influences Hg uptake in adult seals and how Hg is remobilized in their tissues and transferred to their offspring during key physiological processes such as lactation and fasting. They also informed collaborative work on the development of a photometric method to predict body condition of adult harp seals based on external appearance, for use in future studies of the ecology and energy balance of harp seal stocks (National Progress Report Norway 2019).
Norway also works with Russia to monitor and study the harp seals of the Barents Sea/White Sea stock. In 2018, data for assessment of biological parameters and studies on contaminants and ecology (stable isotopes) were collected from 170 harp seal females in the East Ice, with analyses in progress (National Progress Report Norway 2018). It is planned to collect more data during the commercial hunt in 2021 (National Progress Report Norway 2020).
Satellite Tagging
In collaboration with the Greenland Institute of Natural Resources, 26 newborn harp seal pups were equipped with satellite-linked dive recorders during the 2017 breeding season in the West Ice. This provided the first description of the foraging migrations and diving behaviour of young harp seals. The project has also collected valuable oceanographic data from areas where such data is otherwise difficult and expensive to obtain. Harp seals from the West Ice were equipped with satellite tags also in 2018, and data is still being received from these transmitters. Tagging with satellite-based tags was to be attempted in the White Sea in spring 2019 but this was postponed to 2021 (National Progress Report Norway 2018, 2020).
In 2018, three wild-caught female harp seals were instrumented with satellite tags and released into the Barents Sea following 2.5 years in captivity in connection with a controlled validation study on energetics. The tags transmitted for 45, 67 and 162 days, and all three seals exhibited signs of behaviour comparable to wild harp seals (Blanchet et al. 2018).
Monitoring
In 2013 and 2014, information was collected by researchers at the Norwegian Institute of Marine Research to assess efficiency and animal welfare issues in the Norwegian commercial sealing of harp seals in the Greenland Sea. The publication of this analysis is in progress (National Progress Report Norway 2020).
GREENLAND
In Greenland, research is being conducted by the Greenland Institute of Natural Resources. The most recent study was tagging harp seal pups with satellite-linked data-loggers to describe the distribution and behaviour during the first year of their life. This was done in collaboration with Norwegian colleagues (see above), as a part of “The Northeast Greenland Environmental Study Program 2017-1018”, paid by oil license holders in the area. Analysis of the achieved data is ongoing (National Progress Report Greenland 2020).
IN COLLABORATION
Scientists from both Norway and Greenland, as well as from Russia and Canada, participate in the ICES/NAFO/NAMMCO Working Group on Harp and Hooded Seals.
REFERENCES
Andrianov, V.V. (2014). Specific features of the spatial and temporal distribution of the White Sea population of harp seal. Biology Bulletin, 41(8), 690-702. https://doi.org/10.1134/S1062359014080020
Bajzak, C.E., Hammill, M.O., Stenson, G.B. and Prinsenberg, S. (2011). Drifting away: implications of changes in ice conditions for a pack-ice-breeding phocid, the harp seal (Pagophilus groenlandicus). Canadian Journal of Zoology, 89, 1050–1062. https://doi.org/10.1139/z11-081
Bellido, J.J., Cabot, J., Castillo, J.J., Báez, J.C., Martín, J.J., Mons, J.L., Larios, J., Rubia, J. and Real, R. (2009). First record of the harp seal (Pagophilus groenlandicus) extralimital presence in the Mediterranean Sea. Marine Biodiversity Records, 2. https://doi.org/10.1017/S1755267209990959
Blanchet, M.-A., Acquarone, M., Biuw, E.M., Larsen, R., Nordøy, E.S. and Folkow, L.P. (2018). A Life After Research? First release of harp seals (Pagophilus groenlandicus) after temporary captivity for scientific purposes. Aquatic Mammals, 44, 343-356. https://doi.org/10.1578/AM.44.4.2018.343
Carr, S.M., Duggan, A.T., Stenson, G.B. and Marshall, H.D. (2015). Quantitative phylogenomics of within-Species mitogenome variation: Monte Carlo and non-parametric analysis of phylogeographic structure among discrete transatlantic breeding areas of harp seals (Pagophilus groenlandicus). PLoS ONE, 10(8), e0134207. https://doi.org/10.1371/journal.pone.0134207
Committee on Taxonomy. (2016). List of marine mammal species and subspecies. Society for Marine Mammalogy. www.marinemammalscience.org
Corbett, J.J., Lack, D.A., Winebrake, J.J. , Harder, S., Silberman, J.A. and Gold, M. (2010). Arctic shipping emissions inventories and future scenarios. Atmospheric Chemistry and Physics, 10, 9689–9704. https://doi.org/10.5194/acp-10-9689-2010
DFO. (2010). A review of ice conditions and the harp seal Total Allowable Catch (TAC) for 2010. DFO Canadian Science Advisory Secretariat. Sci. Resp. 2010/004. http://www.dfo-mpo.gc.ca/csas-sccs/publications/scr-rs/2010/2010_004-eng.htm
DFO. (2016). Harp Seal and Hooded Seal competitive fleet in Newfoundland and Labrador, Quebec, Gulf and Maritimes Regions. http://www.dfo-mpo.gc.ca/decisions/fm-2016-gp/atl-03-eng.htm Accessed 5 October 2016.
Folkow, L.P., Nordoy, E.S. and Blix, A.S. (2004). Distribution and diving behaviour of harp seals (Pagophilus groenlandicus) from the Greenland Sea stock. Polar Biology, 27, 281-298. https://doi.org/10.1007/s00300-004-0591-7
Frie, A.K., Potelov, V.A., Kingsley, M.C.S. and Haug, T. (2003). Trends in age-at-maturity and growth parameters of female Northeast Atlantic harp seals, Pagophilus groenlandicus (Erxleben, 1777). ICES Journal of Marine Science, 60, 1018-1032. https://doi.org/10.1016/S1054-3139(03)00123-1
Hammill, M.O. and Stenson, G.B. (2010). Abundance of Northwest Atlantic harp seals (1952-2010). DFO Canadian Science Advisory Secretariat. Res. Doc. 2009/114. iv + 12 p. http://www.dfo-mpo.gc.ca/csas-sccs/publications/resdocs-docrech/2009/2009_114-eng.htm
Hammill, M.O. and Stenson, G.B. (2014). Changes in ice conditions and potential impact on harp seal pupping. DFO Canadian Science Advisory Secretariat. Res. Doc. 2014/025. iv + 14 p. http://www.dfo-mpo.gc.ca/csas-sccs/publications/resdocs-docrech/2014/2014_025-eng.html
Hammill, M.O., Stenson, G.B., Doniol-Valcroze, T., and Mosnier, A. (2011). Northwest Atlantic Harp Seals Population Trends, 1952-2012. DFO Canadian Science Advisory Secretariat. Res. Doc. 2011/099. iv + 28 p. http://www.dfo-mpo.gc.ca/csas-sccs/Publications/ResDocs-DocRech/2011/2011_099-eng.html
International Council for the Exploration of the Sea (ICES). (2013). Report of the working group on harp and hooded seals (WGHARP), 26-30 August 2013, PINRO, Murmansk, Russia. ICES CM 2013/ACOM:20. 65 pp.
International Council for the Exploration of the Sea (ICES). (2016). Report of the ICES/NAFO/NAMMCO Working Group on Harp and Hooded Seals (WGHARP), 26-30 September 2016, ICES HQ, Copenhagen, Denmark. ICES CM 2016/ACOM:21. 85 pp. Available at: http://nammco.wpengine.com/wp-content/uploads/2017/01/ices-nammco-nafo-wgharp-report-final.pdf
International Council for the Exploration of the Sea (ICES). (2019). ICES/NAFO/NAMMCO Working Group on Harp and Hooded Seals (WGHARP). ICES Scientific Reports, 1, 72. 193 pp. http://doi.org/10.17895/ices.pub.5617
Johnston, D.W., Bowers, M.T., Friedlaender, A.S. and Lavigne, D.M. (2012). The Effects of Climate Change on Harp Seals (Pagophilus groenlandicus). PLoS ONE, 7(1), e29158. https://doi.org/10.1371/journal.pone.0029158
Kovacs, K.M. (2015). Pagophilus groenlandicus. The IUCN Red List of Threatened Species 2015: e.T41671A45231087. http://dx.doi.org/10.2305/IUCN.UK.2015-4.RLTS.T41671A45231087.en
Kovacs, K.M. and Lydersen, C. (2016). http://www.npolar.no/en/species/harp-seal.html
Lindstrøm U., Nilssen, K.T., Pettersen, L.M.S. and Haug, T. (2013). Harp seal foraging behaviour during summer around Svalbard in the northern Barents Sea: diet composition and the selection of prey. Polar Biology, 36, 305-320.
Atlantic Marine Mammal Commission (NAMMCO). (2004). Report of the NAMMCO Workshop on Hunting Methods for Seals and Walrus. https://nammco.no/assets/Uploads/Report-from-the-Workshop-on-Hunting-Methods-for-Seals-and-Walrus-2004.pdf
North Atlantic Marine Mammal Commission (NAMMCO). (2006). Report of the NAMMCO workshop to address the problems of “struck and lost” in seal, walrus and whale hunting. https://nammco.no/assets/Publications/Hunting-Methods-Committee/Report-of-WS-on-Struck-and-Lost-November-2006.pdf
Øigård, T.A., Haug, T. and Nilssen, K.T. (2014). From pup production to quotas: current status of harp sels in the Greenland Sea. ICES Journal of Marine Science, 71, 537-545. https://doi.org/10.1093/icesjms/fst155
Potelov, V.A., Golikov, A.P. and Bondarev, V.A. (2003). Estimated pup production of harp seals Pagophilus groenlandicus in the White Sea, Russia in 2000. ICES Journal of Marine Science, 60, 1012-1017. https://doi.org/10.1016/S1054-3139(03)00095-X
Richard, P. (2001). Marine mammals of Nunavut. Iqaluit: Qikiqtani School Operations, 98 pp.
Rosing‐Asvid, A. (2008). A new harp seal whelping ground near south Greenland. Marine Mammal Science, 24, 730-736. https://doi.org/10.1111/j.1748-7692.2008.00216.x
Sergeant, D.E. (1991). Harp seals, man and ice. Canadian Special Publication of Fisheries and Aquatic Sciences, 114, 153 p. https://www.arlis.org/docs/vol1/K/24728024.pdf
Sjare, B. and Stenson, G.B. (2002). Estimating struck and loss rates for harp seals (Pagophilus groenlandicus) in the Northwest Atlantic. Marine Mammal Science, 18, 710-720. https://doi.org/10.1111/j.1748-7692.2002.tb01068.x
Sjare, B., Walsh, D., Stenson, G.B. and Benjamins, S. (2005). An update on harp seal (Pagophilus groenlandicus) by-catch estimates in the Newfoundland lumpfish fishery. DFO Canadian Science Advisory Secretariat. Res. Doc. 2005/049. http://www.dfo-mpo.gc.ca/csas-sccs/publications/resdocs-docrech/2005/2005_049-eng.htm
Stenson, G.B. and Hammill, M.O. (2012). Living on the edge: Observations of Northwest Atlantic harp seals in 2010 and 2011. DFO Canadian Science Advisory Secretariat. Res. Doc. 2011/108. iv + 12 p. http://www.dfo-mpo.gc.ca/csas-sccs/Publications/ResDocs-DocRech/2011/2011_108-eng.html
Stenson, G.B., Hammill, M.O. and Lawson, J.W. (2011). How many harp seal pups are there? Additional results from the 2008 surveys. DFO Canadian Science Advisory Secretariat. Res. Doc. 2010/137. iv + 19 p. http://www.dfo-mpo.gc.ca/csas-sccs/Publications/ResDocs-DocRech/2010/2010_137-eng.html
Stenson, G.B., Hammill, M.O., Lawson, J.W. and Gosselin, J-F. (2014). Estimating pup production of northwest Atlantic harp seals, Pagophilus groenlandicus, in 2012. DFO Canadian Science Advisory Secretariat. Res. Doc. 2014/057. http://www.dfo-mpo.gc.ca/csas-sccs/publications/resdocs-docrech/2014/2014_057-eng.html
Stenson, G.B., Buren, A.D. and Koen-Alonso, M. (2016). The impact of changing climate and abundance on reproduction in an ice-dependent species, the northwest Atlantic harp seal, Pagophilus groenlandicus. ICES Journal of Marine Science, 73, 250-262. https://doi.org/10.1093/icesjms/fsv202
Stenson, G.B., Haug, T. and Hammill, M.O. (2020) Harp Seals: Monitors of Change in Differing Ecosystems. Frontiers in Marine Science 7: 569258. https://doi.org/10.3389/fmars.2020.569258.
Stewart, R.E.A., Stewart, B.E., Lavigne, D.M. and Miller, G.W. (1989). Fetal growth of northwest Atlantic harp seals, Phoca groenlandica. Canadian Journal of Zoology, 67, 2147-2157. https://doi.org/10.1139/z89-305
Norwegian Polar Institute – Harp Seal
Institute of Marine Research – Harp Seal (In Norwegian)
Environmental Monitoring of Jan Mayen and Svalbard – Harp seal
Greenland Institute of Natural Resources – Harp Seal
Canadian Geographic Animal Facts – Harp seal
US National Oceanographic and Atmospheric Administration (NOAA) Fisheries – Harp seal