Hooded Seal
Hooded Seal
Latest update: July 2021
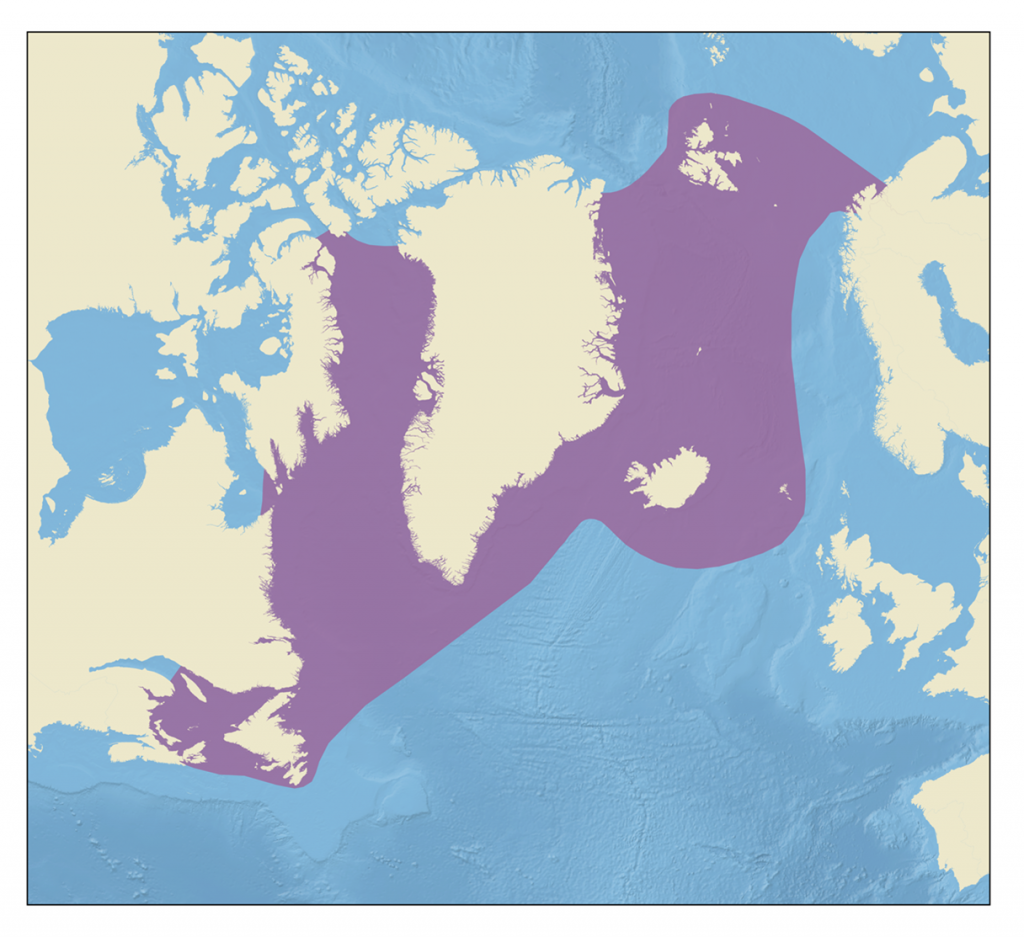
The hooded seal is a large, ice breeding, migratory seal found over the continental shelf regions of the western and central parts of the northern North Atlantic ocean. It is named for the distinctive nasal sac sported by males, which can be inflated to form a bi-lobed hood over the top and front of the head. Mature males also have an elastic nasal septum, which looks like a pink balloon when inflated. These are used in displays during the breeding season and can thus be considered secondary sexual characteristics.
Adult hooded seals are covered in irregularly shaped black spots over a silver-grey background and generally have darkly coloured flippers and face. Hooded seals pups have very distinctive blueish-grey coloured fur on their backs, with lighter coloured fur on their bellies and are therefore commonly referred to as “bluebacks”. They retain this colouration for up to two years, after which the spotted adult pelage begins to appear.
ABUNDANCE
Greenland Sea: ca 76,000
Northwest Atlantic: ca 600,000
DISTRIBUTION
Hooded seals are found in the western and central parts of the northern North Atlantic Ocean.
RELATION TO HUMANS
Previously, hooded seals were hunted in both the Greenland Sea and Northwest Atlantic. Commercial catches in Greenland Sea were banned in 2007 although a low level of catches for scientific purposes continues. Low level catches continue in the Northwest Atlantic.
CONSERVATION AND MANAGEMENT
Scientific advice on stock status and sustainable catch levels is based on the deliberations of the Joint International Council for Exploration of the Sea (ICES) – Northwest Atlantic Fisheries Organisation (NAFO) – NAMMCO Working Group on Harp and Hooded Seals (WGHARP).
In the most recent assessment (2015), the species is listed as ‘Vulnerable’ on the global IUCN Red List. It is listed as ‘Endangered’ on the Norwegian national red list.

© Ann Harvey
Scientific name: Cystophora cristata
Faroese: Klappus
Greenlandic: Natsersuaq
Icelandic: Blôðuselur
Norwegian: Klappmyss
Danish: Klapmyds
English: Hooded seal
LIFESPAN
Hooded seals have an average lifespan of 25-30 years.
SIZE
The largest “true seals” in the North Atlantic, hooded seal males are nearly twice as heavy as females. Males average 2.5 m in length and 300 kg in weight, while mature females are around 2.2 m in length and weigh around 160 kg.
FEEDING
Hooded seals feed primarily on pelagic fish and squid.
PRODUCTIVITY
Males reach maturity at 5-7 yrs, females 3-6 years. Usually, one pup is born per year.
MIGRATION & MOVEMENTS
The species has a close association with Arctic pack ice but also makes long feeding excursions into open water.
GENERAL CHARACTERISTICS
Adult hooded seals are deep-divers, sometimes diving to depths of more than 1,000 m and for up to an hour, where they feed on mollusks, crustaceans and fish. They undertake long seasonal migrations from southern wintering and breeding areas to more northern feeding areas in the summer. Particularly the juveniles are prone to wandering, and are often found far from their normal range. The hooded seal is the largest “true seal” (Family Phocidae) in the North Atlantic.

© Michael Poltermann, IMR
Like many mammals, hooded seals are highly “sexually dimorphic”, which means that males are quite different in size and to some extent body form and colouration than females. Males are nearly twice as heavy as females, averaging about 2.5 m in length and 300 kg at maturity, while females average 2.2 m and 160 kg (Kovacs and Lavigne 1986). Newborn pups are about 1 m in length and weigh 24 kg on average (Kovacs and Lavigne 1992). The pups are fed a rich milk by their mothers for only 3-5 days, the shortest time of any mammal. But during this short period they can nearly double their weight to 42 kg (Bowen 1985)!
Male hooded seals reach maturity in 5-7 years, while females do so in 3-6 years. Their natural longevity is about 30-35 years (Kovacs 2002). Adult hooded seals have no known predators other than polar bears and killer whales, while the young may be eaten by Greenland sharks (Lavigne and Kovacs 1988, LeClerk et al. 2012).
SIZE
Hooded seals show extreme sexual dimorphism, meaning that males differ greatly from females in size and other characteristics. Males are nearly twice as heavy as females, averaging about 2.5 m in length and 300 kg at maturity, while females average 2.2 m and 160 kg. Newborn pups are about 1 m in length and weigh 24 kg on average.
LIFE HISTORY
Females usually give birth to one pup every year. Male hooded seals reach maturity in 5-7 years, while females do so in 3-6 years.
MIGRATION
Hooded seals are an “ice-breeding” seal, and live much of their lives in close association with Arctic pack ice. However they do make long feeding excursions into open water in more southern areas.
FEEDING
Hooded seals can dive to great depths, and feed primarily on pelagic fish and squid, including polar cod (Boreogadus saida), capelin (Mallotus villosus), sand eel (Ammodytes spp.), squid of the genus Gonatus, and larger fish such as Atlantic cod (Gadus morhua), Greenland halibut (Reinhardtius hippoglossoides) and redfish (Sebastes mentella).
LIFESPAN
Hooded seals live about 30-35 years.
Life History and Ecology
The hooded seal (along with harp and ringed seals) is an ice-breeding seal, meaning that females haul out on ice floes to give birth. Seals give birth (called “whelping”) in the same general areas (“whelping grounds”) each year, although the exact location will depend on ice conditions. For the Northwest Atlantic population, there are three whelping grounds: the “Front” (off the coasts of Labrador and north of Newfoundland); the Gulf of St Lawrence (near the Magdalene Islands); and Davis Strait (between Canada and Greenland at about 64° N). For the Greenland Sea population, whelping takes place near Jan Mayen island or farther west towards Greenland (the “West Ice”), depending on ice conditions (Lavigne and Kovacs 1988). Whelping takes place in the latter half of March and is highly synchronized within whelping grounds. All pups are born within a few days of one another (Sergeant 1976).
Hooded seals reach sexual maturity at 4-5 years of age, and most, but not all females produce one pup annually (Frie et al. 2012, NAMMCO 2009a, 2012). Hooded seals live for 25-30 years on average (Kovacs 2002).
Nursing period and mating
Females give birth to a single pup about 1 m in length and weighing about 24 kg (Kovacs and Lavigne 1992). Hooded seals have the shortest lactation (milk suckling) period of any seal, with the pups nursing for only 3 to 5 days! During this brief period the pups can double their weight (Lavigne and Kovacs 1988). The milk of the hooded seal is the richest of any mammal, consisting of over 60% fat and with an energy content of about 560 calories per 100 ml (Haysen 1995). After the nursing period is finished, the mother hooded seals leave their pups, who remain on the ice for a few days until they begin to swim and forage for themselves.
While they are nursing their pups, females are frequently surrounded by one to several males, who compete with one another to mate with the female. This competition consists mainly of display, with males repeatedly inflating their bi-lobed “hoods” and nasal septums. Competition can, however, involve fighting if males are evenly matched and this may result in injury (Kovacs 1992). Mating occurs in the water immediately after the pup is weaned and one male may mate successively with several females (Kovacs 2016). As with all seals, hooded seals have “delayed implantation” (obligate embryonic diapause), in which the fertilized egg remains dormant and does not attach to the uterus for about four months after fertilization. As a result, the females remain pregnant for a full year before giving birth.
Habitat and ecology
DIVING ABILITY

© Michael Poltermann, IMR
Hooded seals are champion divers, able to dive longer and deeper than any other Atlantic seal. They are second only to elephant seals (Mirounga spp.) in their diving ability. Hooded seals can dive for up to an hour and reach depths exceeding 1,500 m, although they more regularly dive for 15 – 20 minutes to depths of 100 – 600 m (Folkow and Blix 1999, Andersen et al. 2013a). Hooded seal pups develop this ability very rapidly and are able to dive for over 15 minutes at only three weeks of age (Folkow et al. 2010). Hooded seals have several adaptations to deep diving. This includes a very high capacity to store oxygen in their blood and muscle tissues (Burns et al. 2007), physiological adaptations to minimize oxygen consumption during diving, and enhanced tolerance of low oxygen levels in the brain (Folkow et al. 2008).
Drift diving
While hooded seals dive mainly to feed, they also carry out a special type of dive called a “drift dive”. In this type of dive, the seal descends rapidly, then the rate of descent abruptly slows down for a period of 10 – 15 minutes, during which the seal sinks slowly. The seal remains nearly motionless during this phase of the dive and appears to be resting. The seal then swims rapidly back to the surface (Andersen et al. 2014). Other deep diving seals, such as elephant seals, also drift dive. It is thought that the seals use drift diving to rest, sleep and/or digest food, while minimizing energy expenditure and remaining safe from predators.
Hooded seals drift dive only at night, at all times of the year. The rate of descent during the passive “drift” phase of the dive depends on the seal’s buoyancy, which is in turn related to their blubber content. This has been used as an indication of the relative condition of the seal. Higher rates of sinking, and thus lower blubber stores, are seen after the breeding and moulting seasons (Andersen et al. 2014).
DIET AND FEEDING
Hooded seals use their excellent diving ability to seek out and capture prey. They feed on a wide variety of fish, crustaceans and mollusks, depending on their location and the available resources.
Greenland Sea
The squid Gonatus fabricii forms a major part of the diet of Greenland Sea hooded seals in the northern part of their distributional range, particularly during the fall and winter months (Haug et al. 2004, 2007). Other major food items for this stock include the pelagic amphipod Parathemisto spp., polar cod (Boreogadus saida), capelin (Mallotus villosus) and sand eel (Ammodytes spp.). Hooded seals also make excursions to more southern waters, particularly around the Faroe Islands and the Irminger Sea south of Iceland. Although their diet in these areas has not been documented, their diving activity in these areas corresponds with the depth distribution of blue whiting (Micromesistius poutassou) and redfish (Sebastes mentella) respectively, which suggests they feed on these species in this part of their range (Folkow and Blix 1995, 1999).

© Michael Poltermann, IMR
Northwest Atlantic
Fish seem to form a larger component of the diet of hooded seals in the Northwest Atlantic, with Greenland halibut (Reinhardtius hippoglossoides), Atlantic cod (Gadus morhua) and redfish being major food items (Hauksson and Bogasson 1997, Hammill and Stenson 2000). Polar cod and shrimp (Pandalus spp.) may form a more important part of the diet in Davis Strait and Baffin Bay (Kapel 1995).
Habitat
While hooded seals are an ice-breeding seal, they are not exclusively or even predominantly denizens of icy Arctic waters. While they require ice floes to haul out on during whelping and moulting periods, at other times of the year they have a rather offshore distribution in deep, often ice-free waters. Tagging studies of hooded seals from the Northwest Atlantic (Andersen et al. 2013b) and Greenland Sea (Folkow et al. 1996) have demonstrated that they prefer areas with water depths of between 200 – 1000 m, generally at the shelf edges and in areas of high bottom relief. In these areas, they do not necessarily dive to the bottom to feed. Rather, they take pelagic fish and invertebrates in the mid-water.
In some areas, males and females have somewhat different habitat requirements, with the larger males diving deeper at the continental shelf edge and at seamounts, while juveniles and females dive to somewhat lesser depths over areas of more uniform bottom topography (Andersen et al. 2013b).
PREDATORS
Hooded seals have few predators other than humans. Polar bears (Ursus maritimus) take mainly pups in some whelping areas, but these do not form a major component of their diet. Killer whales (Orcinus orca) hunt hooded seals in some areas, but the extent of this predation is not known (Kovacs 2016). The Greenland shark (Somniosus microcephalus), a large, slow moving species common in Arctic waters, may also hunt hooded seals, but while the extent of this predation is not known, it is likely to be small (LeClerk et al. 2012).
Distribution and Habitat
Hooded seals are fast swimmers and capable divers. They haul out of the water infrequently except during the whelping and moulting periods (Folkow et al. 1996, Andersen et al. 2013b). While they occupy specific and spatially constrained whelping and moulting areas at certain times of the year, their distribution during other periods is quite widespread and variable.
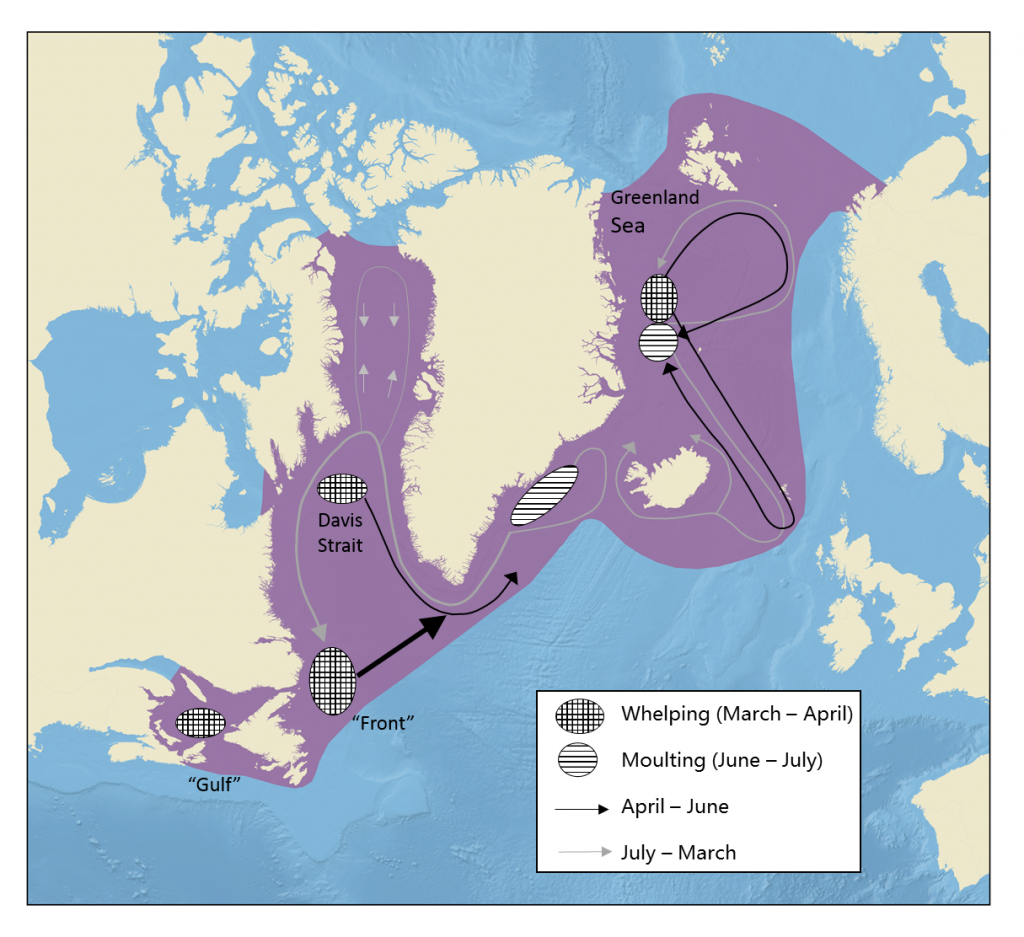 The general distributional cycle of hooded seals.
The general distributional cycle of hooded seals.
Northwest Atlantic stock
Northwest Atlantic hooded seals pup in the latter half of March at the “Front” (off the coasts of Labrador and north of Newfoundland); the Gulf of St Lawrence (near the Magdalene Islands); and the Davis Strait (between Canada and Greenland at about 64° N). Of the three whelping grounds, the Front is by far the largest and is used by over 90% of this stock. After whelping, the seals disperse to the continental shelf edges around Newfoundland and Labrador, Davis Strait between Labrador and southern Greenland, and off the coast of southeast Greenland.
Interestingly, Northwest Atlantic hooded seals do not enter the eastern Canadian Arctic at this time of year (Andersen et al. 2013b). Seals from this stock moult on thick ice flows off southeast Greenland in July. The exact location of the moulting grounds varies from year to year depending on the availability of suitable ice habitat. After moulting, Northwest Atlantic hooded seals again disperse to their feeding grounds, occupying the continental shelf edge off Newfoundland and Labrador, central and western Baffin Bay and Davis Strait, and southwest and southeast Greenland. During these periods, the seals remain at sea almost continuously, sometimes travelling long distances but often remaining in certain areas (presumably good feeding grounds), for extended periods of time (Andersen et al. 2013b).
Greenland Sea stock
The smaller Greenland Sea stock of hooded seals also has a somewhat more constrained distribution and their migratory pattern is quite different from that of the Northwest Atlantic population. Unlike that population, Greenland Sea hooded seals tend to be “based” off the East Greenland coast, near their moulting and whelping areas. From here they make repeated excursions (often lasting one to three months) to distant feeding areas (Folkow et al. 1996). Feeding areas include the entire Greenland and Norwegian Seas (particularly to the north of Iceland and Jan Mayen Island), the seas around the Faroe Islands extending as far south as Ireland, and the Irminger Sea south of Iceland. These feeding excursions are not coordinated and a segment of the population is always present off eastern Greenland, with most animals returning there for one to several weeks between excursions (Folkow et al. 1996, 2010).
Migration
Males, females and juveniles follow roughly the same migratory pattern (Folkow et al. 1996, 2010, Andersen et al. 2013b). However, in some years, juveniles are found in areas far beyond their normal distributional range. For example, strandings of mainly juvenile hooded seals became increasingly common in the Gulf of Maine in the late 1990s and early 2000s (Harris et al. 2001). Juvenile hooded seals have been recorded in places as far south as Antigua in the west and the Canary Islands in the east (Mignucci-Giannoni et al. 2002). Most of the juvenile seals found in these areas are emaciated and in poor condition. The reasons why juveniles end up so far from their normal range in certain years is not understood, but may be associated with sparse sea ice in some years.
Stock Definition
To a biologist, a stock is a sub-population of a species that is largely reproductively isolated from others of the same species. As a result of this reproductive isolation, stocks can usually be differentiated genetically if they have been isolated for a sufficient length of time. Other means of stock differentiation include morphometrics (body size and shape), concentrations of pollutants or rare elements in tissues, behaviour (including vocal dialects), and patterns of seasonal movement. A key feature of this concept is that the hunting and possible depletion of one stock should have little or no effect on a neighbouring stock.
Mixing and shifting
While hooded seals have a continuous distribution that covers most of the northern North Atlantic, they do form discrete, widely separated aggregations for whelping and mating in March and for moulting in July. It is not known to what degree, if any, there is mixing between these whelping areas, but tag returns suggest that most seals utilize the same breeding areas repeatedly. However, at other times of the year, the distribution of hooded seals from all areas overlaps. In some years, the moulting areas may also overlap.
Sergeant (1974, 1976) suggested that apparent fluctuations in the availability of hooded seal pups at the Front were too great to be changes in actual abundance, which indicated that hooded seals might shift between whelping areas depending on ice conditions or other factors. For example, despite considerable effort, hooded seal catches at the Front were extremely low in the 1930s, but rebounded shortly thereafter. A possible explanation is that seals may have shifted their reproduction to the Davis Strait whelping area during this period, which would indicate that there is mixing between whelping areas and therefore stocks.
Genetics
Historically, hooded seals have been divided into two populations: the Northwest Atlantic stock (including the Gulf, Front and Davis Strait whelping grounds) and the Greenland Sea stock (including the Jan Mayen/West Ice whelping ground). This separation was based primarily on the wide separation of the West Ice whelping ground from the other three, and the fact that the main hunting areas (the Front for the Northwest Atlantic, and the West Ice for the Greenland Sea) were also widely separated.
However, a genetic study (Coltman et al. 2007) found no significant differences in either mitochondrial or nuclear DNA among seals from the four whelping grounds. This suggests either that there is enough mixing among whelping grounds to prevent genetic differences from developing, or that there has been insufficient time for such differences to develop.
The wide dispersal of juvenile hooded seals (Mignucci-Giannoni et al. 2002) is a probable mechanism whereby genetic exchange between breeding areas might occur.
Management
Despite the lack of evidence for stock separation, continuing to manage hooded seals as two separate stocks is considered to be a conservative and prudent approach. Management as one North Atlantic stock could lead to depletion of one or more stocks if stock structure actually does exist. For example, if most of the hunt was concentrated in an area occupied by a small stock. Satellite tagging data also suggests that the two stocks are separated at most times of the year (Folkow et al. 1996, Andersen et al. 2013b), and hunting activities are widely separated. Moreover, at present, the two putative stocks differ greatly in abundance, trend in abundance and conservation status, with the Greenland Sea stock depleted and decreasing, and the Northwest Atlantic stock stable or increasing. This is further support for the suggestion that different management approaches are needed for the two stocks.
Current abundance and trends
COUNTING HOODED SEALS
The total number of hooded seals would be nearly impossible to count directly because the seals are widely distributed across the North Atlantic and Arctic Oceans at most times of the year and they spend most of their time underwater. Although they do congregate during the whelping and moulting periods, not all of the population is present at the surface at any one time or place. Therefore direct surveys of the whelping grounds and counting seal pups on the ice are used in combination with population models to estimate the total number of seals.
Methods
Hooded seal surveys use a combination of visual and photographic methods. Visual reconnaissance surveys, using helicopters and fixed wing aircraft, are used to locate and map the whelping patches. Photographic survey transects are then flown over the patches. Pups are counted on the photos by experienced readers, and the counts are verified by repeated readings. The pups are classified by “stages” according to their apparent age, from newborn to weaned pup. These data are used in combination with information from tagged pups to estimate the proportion of pups that are on the ice, and not in the water, and thus “available” to be seen at any one time. The corrected pup density on the photographic transects is applied to the entire survey area using standard strip transect methodology.
Estimates of pup production are extended to estimates of the total population using population models. These models incorporate data on pregnancy rate at age, natural mortality and catch at age to calculate the number of adult seals that would produce the number of pups observed in the survey (Hammill and Stenson 2006, Salberg et al. 2008).
GREENLAND SEA STOCK
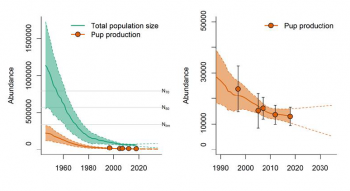
Modeled population trajectories for Greenland Sea hooded seal pups and adults (full lines), 95% confidence intervals (shaded areas) and future projections (dashed lines). Observed pup production estimates indicated by filled circles. (ICES 2019)
Norwegian scientists have surveyed this stock in 1997, 2005, 2007, 2012 and 2018 (NAMMCO 2006a, 2009a, 2012, 2013, Salberg et al. 2008, Øigard et al. 2014, ICES 2019). Estimated pup production has declined steadily and substantially over that period, from 28,100 (95% CI: 16,000 – 40,000) in 1997 to 12,977 (95% CI: 9867 – 17,067) in 2018. The magnitude of the decline is likely even greater than this because in contrast to recent surveys, the 1997 estimate was not corrected for biases due to pups being in the water, reader error, or pups born outside the main concentration area (Øigard et al. 2014). Similarly, the estimated total number of seals has declined from 102,000 (95% CI: 57,000 – 147,000) in 1997 to 76,623 (95% CI: 58,299 – 94,947) in 2018.
Øigard et al. (2014) used survey estimates along with historical catch data and reproductive data to model the historical population trajectory of the stock from 1946. This suggested that the stock has declined from about 1.4 million animals in 1946 to less than 100,000 now, and that it will continue to decline even if there is no catch.
NORTHWEST ATLANTIC STOCK
The Northwest Atlantic stock includes three whelping patches, one of which, the Davis Strait, is widely separated from the Gulf and Front patches. This makes surveying all three areas in the short pupping season logistically challenging. On the other hand, the Front whelping patch accounts for more than 90% of the total number so surveys covering that area alone will account for most of the population.

Estimated abundance of Northwest Atlantic hooded seals, 1865-2006. DFO Canada Scientific Research Report 2006-2008
Surveys using roughly comparable methods have been carried out in the area between 1984 and 2005 (Hammill and Stenson 2000, 2006). Only one of these, in 2005, covered all three whelping patches in a single season. Pup production at the Front had increased steadily from 62,400 in 1984 to 107,000 in 2005. Estimates of pup production in the Gulf were much smaller, ranging from about 2,000 to 8,700. The Davis Strait patch had been surveyed only twice, in 1984 and 2005, and pup production estimates varied greatly from 19,000 to 3,300 in the more recent survey. The total population size, including pups and adults was estimated as 593,500 (95% CI: 465,600 – 728,300) in 2005 (Hammill and Stenson 2006).
Combining these survey estimates with historical catch and reproductive data to model the trend in the population indicates that the stock was at a minimum size of less than 375,000 seals in 1984 (when hunting at the Front largely ceased), increasing to the present estimated level of nearly 600,000 animals, an increase rate of just over 2% per year (Hammill and Stenson 2006).
Stock Status
Assigning a “status” to a particular stock of animals is a complex and sometimes controversial exercise that requires, among other things, some knowledge of the historical abundance trend of the stock, an assessment of the reliability of that knowledge, and clearly stated management/conservation goals with respect to the stock. Since there is no universal definition of stock status used by all organizations and countries, it is important to clearly define how stock status will be interpreted before an assessment is made.
The management framework
A management framework for harp and hooded seals that clearly defines stock status has been adopted by NAMMCO member countries and by Canada (NAMMCO 2004a, 2005, Hammill and Stenson 2007). The framework distinguishes between “data rich” and “data poor” stocks based on defined criteria regarding the quality and age of the available information on abundance and biological parameters. For example, data rich stocks must have at least five abundance estimates spanning a period of 10-15 years with surveys separated by 2-5 years, and the most recent abundance estimate should be no more than five years old. As there will be more uncertainty about the status of data poor stocks, the criteria used to assign status are different.
Data rich stocks
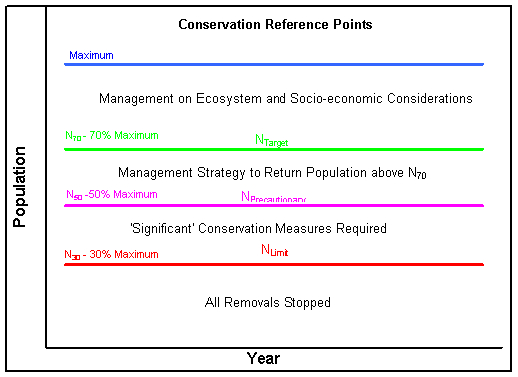
For data rich stocks, the framework establishes numerical population reference levels (see below), the most important of which are the precautionary level, for hooded seals set at 70% of the maximum stock size observed (Nmax) and therefore called N70, and the limit level, for hooded seals set at 30% of the maximum stock size observed (N30). The abundance levels of these reference points may be species
Credit: DFO (http://www.dfo-mpo.gc.ca/fm-gp/seal-phoque/reports-rapports/mgtplan-planges20112015/mgtplan-planges20112015-eng.htm)
specific and also depend on management objectives for the stock. Stocks above N70 are of least conservation concern and management goals can be set to increase, stabilize or decrease the population as long as it stays above N70. If a stock is below N30, the stock is considered to be of greatest conservation concern so no hunting can be allowed and management must have the goal of increasing stock size to above N70. Other reference levels may be set between these two levels, which may allow for limited hunting but always with the goal of increasing stock size above N70.
Data poor stocks
For data poor stocks, only a limit level is required, below which no catch is allowed. In addition, catch levels must be set in a more precautionary way that will allow the population to increase towards Nmax. Data poor stocks can become data rich as additional surveys are done and more information about the stock becomes available.
Other ways of classifying stocks
Other organizations classify the status of stocks in different ways. For example, the International Union for Conservation of Nature and Natural Resources (IUCN) uses specific criteria to assign species to one of nine classifications ranging from “Least Concern” to “Vulnerable” to “Extinct”.
GREENLAND SEA STOCK
At present, under the management framework described above, the Greenland Sea stock can be considered “data rich” as there have been several recent surveys including one in 2018, as well as recent information on other biological parameters. The stock size, estimated at 76,623 (95% CI: 58,299 – 94,947) in 2019 (ICES 2019) is currently well below the N30 limit level (30% of the maximum estimated historical population size of over 1 million animals). Therefore following the precautionary framework developed for stock status assessments, no commercial catches should be taken from this stock.
There is a forecast that the stock will continue declining even without hunting (Øigard et al. 2014). This stock is therefore of maximum conservation concern and the Joint ICES/NAFO/NAMMCO Working Group on Harp and Hooded Seals (WGHARP) has therefore recommended that there be no commercial hunt of this stock, although low level hunting for subsistence and scientific purposes has been permitted (ICES 2019).
It is likely that this stock was initially reduced by commercial hunting by Norwegian and Russian sealers, which began in earnest in the 1920s. Catch levels ranged between 40 – 60 thousand seals per year until the late 1950s, when management measures were taken to reduce the hunt (NAMMCO 2006a). Hunt was gradually reduced and averaged less than 5,000 animals per year, mainly pups, from 1990-2005. Hunting was terminated except for subsistence and scientific purposes in 2007.
Continued decline due to reductions in ice cover?
The decline in the stock has apparently continued despite the reduction in catches and its near cessation in 2007, however the reasons for this are unclear. Pregnancy rates in females do not appear to have declined and have been comparable to those of Northwest Atlantic hooded seals (NAMMCO 2013, Frie et al. 2012). Hooded seals are dependent on stable ice platforms for whelping and moulting, and there has been a substantial reduction in ice cover in the area in recent decades, with seals being forced to whelp and moult closer to the Greenland coast (Øigard et al. 2014). This might have resulted in increased mortality of pups and/or adults, or forced the seals to find a new breeding area that has not been covered by the surveys.
It is also possible that the reduced ice cover has made hooded seals more vulnerable to predation by polar bears and killer whales (Kovacs et al. 2011). Competition with commercial fisheries for such valuable species as cod, redfish and Greenland halibut could also be a contributing factor.
NORTHWEST ATLANTIC STOCK
Under the stock status system described above, Northwest Atlantic hooded seals are a “data poor” stock as there have been no surveys covering all areas carried out in recent years and little new data on biological parameters. Combining survey estimates with historical catch and reproductive data to model the trend in the population indicates that the stock was at a minimum size of less than 375,000 seals in 1984 (when hunting at the Front largely ceased), increasing to the estimated present level of nearly 600,000 animals, an increase rate of just over 2% per year (Hammill and Stenson 2006). This indicates that the Northwest Atlantic stock of hooded seals was increasing in numbers until 2005, however the lack of recent data on the stock size is of concern.
Historical catches
Commercial catches of hooded seals in the Northwest Atlantic has been very high historically, reaching over 60,000 in some years in the early 20th century. Seals (mainly pups) were hunted primarily at the Front but also in the Gulf. Between 1965 and 1982, catches at the Front ranged between 10 – 15 thousand animals per year, with additional seals taken in the Gulf, off West Greenland, and in the moulting area off southeast Greenland. Hunts were reduced substantially after 1983 (Stenson et al. 1991). It is likely that this high level of take led to a reduction of the hooded seal stock, and that the stock is still recovering.
Decline in reproductive rate
There is evidence that the reproductive rate has declined since the 1970s (Frie et al. 2012). This could be due to intraspecific competition in an expanding population, declines in the abundance of prey species such as cod and redfish, competition with the more abundant harp seal, or some combination of these factors.
Whelping areas
The Northwest Atlantic stock includes three whelping areas, with the Front accounting for about 90% of pup production. Pup production in the Gulf seems quite variable, ranging from 2000-9000, and may depend on the availability of suitable ice in the area (Hammill and Stenson 2006). The Davis Strait patch has been surveyed only twice, in 1984 and 2005, and pup production estimates varied greatly from 19,000-3,300 in the more recent survey. Given the uncertainty surrounding stock relationships for this species (Coltman et al. 2007), caution is required to avoid overexploitation of these smaller population units.

© Michael Poltermann, IMR
MANAGEMENT FRAMEWORK
NAMMCO provides scientific advice on stock status and sustainable takes, and proposals for conservation and management to member governments, including Norway and Greenland. The NAMMCO Scientific Committee bases its advice on the deliberations of the Joint International Council for Exploration of the Sea (ICES) – Northwest Atlantic Fisheries Organisation (NAFO) – NAMMCO Working Group on Harp and Hooded Seals (WGHARP). A management framework for harp and hooded seals that embodies the precautionary approach to fishery management and clearly defines stock status has been adopted by NAMMCO member countries and by Canada (NAMMCO 2004a, 2005, Hammill and Stenson 2007, DFO 2016). More detailed information on the management framework is given above under “Stock Status.”
NORTHWEST ATLANTIC STOCK
The Northwest Atlantic stock has historically been hunted by Canadian and Norwegian commercial sealers at the Front and the Gulf, Norwegian sealers at the moulting grounds off Southeast Greenland, by West and East Greenlanders, and to a very small extent by Canadian Inuit. The first management measures (opening and closing dates for the commercial catch), were introduced in the 1950s (Sergeant 1976). Hunting of hooded seals in the Gulf was banned in 1965 due to concerns about over-hunt of this small sub-group. The first Total Allowable Catch (TAC) for Canadian waters was introduced in 1974, and TACs have been in place for hooded seals in Canada since that time (Stenson 2006). Prior to 2007, the TAC for hooded seals was set at 10,000, however new data on the status of the population (Hammill and Stenson 2006) saw this reduced to 8200 in 2007. Hooded seals have not been assessed since 2006 and therefore no changes in the TAC have been implemented since then. The TAC has also not been formally announced since 2016 (ICES 2019).
In 1983, the European Economic Community banned the import of hooded seal pup (blueback) skins, largely destroying the market for these pelts. This led to a ban on the commercial hunting of bluebacks in Canada in 1987, and a limitation on the hunt to vessels of less than 65 feet in length. Commercial hunts since then have been relatively low, generally under 1,000 except for exceptionally high catches from 1996 – 1998 when there was a subsidy for seal meat (Stenson 2006).
Estimating quotas
Under the management framework used by Canada and NAMMCO member countries, Northwest Atlantic hooded seals are a “data poor” stock as few surveys have been conducted, and none since 2005. Available information indicates that the Northwest Atlantic stock of hooded seals was increasing in numbers until 2005, and is well above the N30 limit level. However the lack of recent data on the stock size is of concern.
Because of its data poor status, quotas for this stock are estimated using the very conservative Potential Biological Removal (PBR) approach (Hammill and Stenson 2007). This approach uses a minimum estimate of population size and rate of increase to calculate a removal rate that has a high probability of allowing the population to grow. The last calculated PBR for this stock is 32,000 animals, which must include Greenlandic as well as Canadian catches and also account for animals that are struck and lost or taken as by-catch. Taking these other sources of mortality into account lead to a TAC of about 8,000 seals for Canada (Hammill and Stenson 2007).
Under the Canadian “Atlantic Seal Management Strategy”, adopted in 2003, the Minister of Fisheries and Oceans can vary quotas to account for new information that might be obtained on the status of the population, changing environmental conditions, or changes in catch levels in Arctic Canada and Greenland. For the past several years the commercial quota for hooded seals has been set at 8,200 animals, but takes have generally been less than this.
Regulations
Seasons and hunting areas for hooded seal hunt in Canada are set out in the Marine Mammal Regulations (MMR) under Canada’s Fishery Act. The MMR also specify the type and specifications that can be used to take seals. For example, the MMR specifies the dimensions of allowable clubs and hakapiks, and the type of firearms that can be used to hunt seals. The MMR also specify measures that must be taken to ensure the seal is dead before skinning.
Hooded seals from this stock area are hunted by Canadian Inuit in Labrador, Nunavik and Nunavut, and by West and East Greenlanders. Catches by Canadian Inuit for subsistence are not limited but are very low. The Ministry of Fisheries, Hunting and Agriculture is responsible for regulating seal hunting in Greenland. The municipalities set local regulations on seal hunting. Seals can be hunted all year round, provided that hunters have a permit. There are no quotas for seals in Greenland. However, the permits are used to control and monitor the hunt, as hunters are required to report their annual catches. Compliance with quotas and other regulations is monitored by wildlife officers at the local level (MFHA 2012).
GREENLAND SEA STOCK
Historically, Norway and Greenland have participated in the hunt of hooded seals in the Greenland Sea. Russia also participated in the commercial hunt between 1958 and 1994 (NAMMCO 2006a), and very small numbers of hooded seals are occasionally taken in Iceland (Thordarson et al. 2007).
At present, under the management framework used by NAMMCO, the Greenland Sea stock can be considered “data rich” as there have been several recent surveys including one in 2018, as well as recent information on other biological parameters. The stock size is less than 30% of the maximum estimated historical population size of over 1 million animals, and therefore below the N30 limit level at which all hunt must cease. This stock is therefore of maximum conservation concern and the ICES/NAFO/NAMMCO Joint Working Group on Harp and Hooded Seals has therefore recommended that all hunting of this stock (except for subsistence and scientific purposes) cease (ICES 2019).
It is likely that this stock was initially reduced by commercial hunting by Norwegian and Russian sealers, which began in earnest in the 1920s. Catch levels ranged between 40 and 60 thousand seals per year until the late 1950s, when management measured were taken to reduce the catch (NAMMCO 2006a). Hunting was gradually reduced and averaged less than 5,000 animals per year, mainly pups, from 1990-2005. Commercial hunting was stopped in 2007.
Regulations
The Ministry of Fisheries, Hunting and Agriculture is responsible for regulating seal hunting in Greenland. The municipalities set local regulations on seal hunting. Seals can be hunted all year round provided that hunters have a permit. There are no quotas for seals in Greenland. However, the permits are used to control and monitor the hunt as hunters are required to report their annual catches. Compliance with quotas and other regulations is monitored by wildlife officers at the local level (MFHA 2012).
Seal hunting in Norway is managed under the Ministry of Trade, Industry and Fisheries, which establishes regulations governing seasons, specifications of hunting weapons and hunting methods. Sealing vessels must have a public veterinary inspector on board to ensure compliance with rules and regulations concerning sealing. Sealers are required to complete courses on the rules and pass shooting tests before they are allowed to take part in sealing expeditions. In the past, Norway has provided subsidies to seal hunters. While these were stopped in 2014, they were reintroduced at a significantly lower level for the 2016 hunt and have remained in place since that time (ICES 2019). Under Norwegian regulations, after being shot the seal must be struck with a club or hakapik, then bled. Sealers are also required to take a one-day training course annually (NAMMCO 2004b).
Hunting and Utilisation
Hooded seals have been hunted for thousands of years by indigenous communities in Arctic Canada, Newfoundland and Labrador, and Greenland. Commercial exploitation of the Greenland Sea stock began in the 18th century and the earliest recorded voyage for seals took place in 1720. During the 18th and 19th centuries, ships from Germany, Holland, Denmark, Britain and later Norway took part in this hunt (Sergeant 1991). The Newfoundland commercial hunt developed in the late 19th century. Canadian companies participating in the hunt were largely replaced by Norwegian companies by the mid-20th century, although these companies continued to employ local people (Sergeant 1976).
Generally, the commercial hunt can be divided into two types: distant water hunting conducted by ocean going vessels (targeting whelping and moulting areas far from land) and land based hunting targeting whelping areas close to land. The Greenland Sea stock was targeted by distant water fleets as the whelping and moulting patches are not accessible from land, while shore based hunters targeted seals in the Gulf and at the Front in some years.
Prior to the 1930s, most commercial sealing targeted adult seals for oil and leather. The oil was used for lighting and machine lubrication. With the collapse of the seal oil market due to replacement by petroleum products, and the development of better methods for preserving pelts, the emphasis shifted to fine furs for the garment trade. This in turn led to the targeting of young seals, particularly young of the year hooded seals (bluebacks), which had the most valuable pelt of all seals (Sergeant 1976).
EU SEALSKIN BAN AND THE INUIT EXEMPTION
In 1983, the European Economic Community imposed an import ban on all sealskin products made from seal pups, including young hooded seals (bluebacks) (Kovacs 2016). The ban, which was initially for two years, has been extended since then. In 2009, this ban was extended to include all seal products. Exceptions to the ban include products resulting from Inuit/Indigenous hunts; products for the sole purpose of sustainable management of marine resources; and products for the personal use of travelers to the EU. Furthermore, the ban does not apply to seal products trans-shipped through the EU to non-EU destinations (DFO 2016).
In order for seal products to be exempted from the ban and placed on the market in the EU, the Inuit or Indigenous hunt must meet certain conditions. The hunt must:
- be traditionally conducted by the community
- contribute to the community’s subsistence in order to provide food and income and not be primarily conducted for commercial reasons
- pay due care to animal welfare, while taking account of the community’s way of life and the subsistence purpose of the hunt.
The effect of these trade barriers has been to significantly reduce the market for seal skins and thereby to depress prices. This has reduced the economic viability of commercial seal hunting to the point where it has been largely abandoned in Norway and substantially reduced in Canada and Greenland (DFO 2016, MFHA 2012).
SEALING IN GREENLAND
Hunters in Greenland target hooded seals primarily during the open water season, hunting them from small boats using rifles. Hooded seals appear in April and new-born seal pups can sometimes be found on the floe edge or in the drifting ice (NAMMCO 2004b). Seals are shot then retrieved using a hook on a pole or a small harpoon.
Animals from the Northwest Atlantic stock are taken in West and southeast Greenland, and this stock accounts for over 95% of the total catch. Animals from the Greenland Sea stock are taken only in Ittoqqortoomiit in northeast Greenland (MFHA 2012). From 1993 to 2009, total catches varied from about 2,000 to just over 8,000 per year, averaging 5,900 over the period. Of these less than 200 per year were taken from the Greenland Sea stock (MFHA 2012).
Sealing is integrated into a mixed subsistence and commercial economy in Greenland. On average, skins from about half the catch are sold commercially, and only a very small proportion of these come from hooded seals. The remainder are used domestically for the production of clothing and other crafts. Sealskins are purchased by Great Greenland A/S which is owned wholly by the Greenland Government. The company maintains trading stations and storage depots in 46 communities in Greenland. Due to the recent low prices for seal skins, supplying the remaining market is not feasible without subsidies (MFHA 2012).
Seals are a major source of meat in Greenland. While much of the meat is used domestically or shared in the community, part of the catch is sold in the open-air markets (Greenlandic Kalaalimineerniarfik, Danish brædtet), which are present in every village and town. This provides a welcome source of income for hunters.
SEALING IN NORWAY
Commercial exploitation of the Greenland Sea stock began in the 18th century, and the earliest recorded voyage for seals took place in 1720. During the 18th and 19th centuries, ships from Germany, Holland, Denmark, Britain and later Norway took part in this hunt (Sergeant 1991). By the 1920s, this hunt was completely dominated by Norway (Sergeant 1976).
Norwegian sealers must travel a long way (500 to 800 nm) to reach the whelping areas west of Jan Mayen or the moulting areas off east Greenland. this means the hunt is limited to relatively large ocean-going fishing vessels. Sealing is only a seasonal activity for these fishermen, who concentrate on fishing for the remainder of the year (NAMMCO 2004b). Catch levels ranged between 40 to 60 thousand seals per year until the late 1950s, when management measured were taken to reduce the hunt (NAMMCO 2006a). In 2007, hunting for hooded seals was banned except for scientific research purposes, so no commercial hunting of hooded seals has taken place since that time. Total catches for scientific purposes have been 17 (including 14 pups) in 2017, 17 (including 9 pups) in 2018, and 23 (including 14 pups) in 2019 (ICES 2019).
In addition to taking seals on the whelping grounds, Norwegian hunters historically targeted the moulting area off East Greenland from 1945 to 1960. Annual catches in this area ranged from 1,500 to 48,000 per year over the period and averaged 14,500 (Stenson 2006). This catch was likely a combination of seals from the Northwest Atlantic and Greenland Sea stocks.
SEALING IN CANADA
The Newfoundland and Gulf commercial hunts were developed in the late 19th century by local interests. Canadian companies participating in the hunt were largely replaced by Norwegian companies by the mid-20th century, although these companies continued to employ local people (Sergeant 1976). While harp seals have always been the main target of the hunt, hooded seals were also taken. Generally, the commercial hunt can be divided into two types: distant water hunting conducted by ocean going vessels (targeting whelping areas far from land and staying on the grounds for several days at a time), and land based hunting targeting whelping areas close to land.
Historically
Prior to 1930, most hunting was for adult seals for the oil and leather trade. With the demise of the market for seal oil, emphasis shifted to providing fine furs for the garment trade. This in turn led hunters to take mostly seal pups and a higher proportion of hooded seal pups (bluebacks), which had the most valuable pelt (Sergeant 1976).
Commercial catches of hooded seals in the Northwest Atlantic were historically very high – over 60,000 in some years in the early 20th century. Seals (mainly pups) were hunted primarily at the Front but also in the Gulf. Prior to the imposition of quotas in 1974, Canadian catches were highly variable, ranging from a couple of hundred to more than 25,000. Between 1965 and 1982, the catch at the Front ranged from 10,000-15,000 animals per year, with some additional seals taken in the Gulf.
Recent years
Catches were reduced substantially after the 1983 imposition of the European trade ban on pelts from young seals and the Canadian ban on the taking of bluebacks in 1987 (Stenson et al. 1991, Stenson 2006). Following these bans, catches dropped to only a few hundred annually, except in 1991 (6,000) and in 1996 – 1998 when a subsidy for seal meat led to catch of 26,000, 7,000 and 10,000 respectively (Stenson 2006). Catches of hooded seals were 12 in 2017, 79 in 2018 and 30 in 2019, all of which were animals older than 1 year.
A small number of hooded seals, fewer than 10 in most years, are also hunted by Canadian Inuit living in Nunavut (Stenson 2006).
The economic value of the seal hunt has declined greatly since 2006 and there is currently virtually no market for the pelts of adult hooded seals (DFO 2016).
Methods
In Canada, the typical commercial sealer is a fisherman who uses his or her vessel to fish for other species at other times of the year. In the past, sealing provided a welcome source of income at an otherwise inactive time of year (NAMMCO 2004b). Sealing is limited to small vessels (<35 ft) and longliners up to 65 ft in length. Small vessels have crews of 2 – 5, operate from shore and land their catch daily, while larger vessels might stay out for several days.
Commercial sealers require a licence. To become a professional sealer, an assistant sealer must apprentice under a professional for two years (NAMMCO 2004b). Currently however there is a freeze on the issuance of new sealing licenses (DFO 2016).
Almost all seals taken at the whelping patches are shot on the ice using rifles or shotguns. A “three-step” process is proscribed in the regulations to ensure that seals are killed quickly and humanely. The first step is striking the seal with a rifle or shotgun, or a hakapik or club of prescribed dimensions. Secondly the sealer must palpate the cranium to ensure that it is crushed, and strike the animal again if it is not. Thirdly, the animal is bled for at least one minute by severing the carotid artery (NAMMCO 2009b, DFO 2016).
STRUCK AND LOST
“Struck and Lost” occurs when an animal is hit (struck) by a weapon, such as a rifle bullet or harpoon, but is not retrieved by the hunter. The injured animal may survive or die, depending on the severity of the injury (NAMMCO 2006b). Even if animals survive, their reproductive contribution to the population may be diminished. Struck and lost is a source of mortality, as direct hunt is, and should therefore be incorporated into estimates of removals and population models. However, struck and lost is very difficult to estimate as it generally requires intensive monitoring of hunting activities by an independent observer (NAMMCO 2006b).
Struck and lost rates are very low for seals shot on the ice in the commercial hunt, ranging from 0 to 2% of struck animals. Loss rates are higher for those struck in the water, ranging up to 10% in some hunts (NAMMCO 2004b).
Struck and lost rates in the Greenlandic open water hunt for hooded seals has been a matter of some controversy. Current population models assume a loss rate of 50%, which is considered too high by most Greenlandic hunters (Hammill and Stenson 2006, NAMMCO 2006b). A questionnaire suggested that the average loss rate for Greenlandic hunters was about 22% (NAMMCO 2006b), and confirmed that loss rates were highest in May and June, when seals have a lower blubber content, are less buoyant and sink before they can be retrieved.
Catches in NAMMCO member countries since 1992
| Country | Species (common name) | Species (scientific name) | Year or Season | Area or Stock | Catch Total | Quota (if applicable) |
|---|---|---|---|---|---|---|
| Greenland | Hooded seal | Cystophora cristata | 2023 | Northwest Atlantic | 930 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2023 | Greenland Sea | 1 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2023 | Total | 931 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2022 | Northwest Atlantic | 943 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2022 | Greenland Sea | 1 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2022 | Total | 944 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2021 | Northwest Atlantic | 1043 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2021 | Greenland Sea | 126 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2021 | Total | 1169 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2020 | Greenland Sea | 6 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2020 | Northwest Atlantic | 903 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2020 | Total | 909 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2019 | Greenland Sea | 334 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2019 | Northwest Atlantic | 1271 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2019 | Total | 1605 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2018 | Ittoqqortoomiit | 283 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2018 | West and Southeast | 709 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2018 | Total | 992 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2017 | Ittoqqortoomiit | 0 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2017 | West and Southeast | 1585 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2017 | Total | 1585 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2016 | Ittoqqortoomiit | 1 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2016 | West and Southeast | 1518 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2016 | Total | 1519 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2015 | Ittoqqortoomiit | 0 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2015 | West and Southeast | 1949 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2015 | Total | 1949 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2014 | Ittoqqortoomiit | 1 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2014 | West and Southeast | 1848 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2014 | Total | 1849 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2013 | Ittoqqortoomiit | 0 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2013 | West and Southeast | 1520 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2013 | Total | 1520 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2012 | Ittoqqortoomiit | 6 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2012 | West and Southeast | 1701 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2012 | Total | 1707 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2011 | Ittoqqortoomiit | 9 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2011 | West and Southeast | 2060 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2011 | Total | 2069 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2010 | Ittoqqortoomiit | 6 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2010 | West and Southeast | 2119 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2010 | Total | 2125 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2009 | Ittoqqortoomiit | 1 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2009 | West and Southeast | 1985 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2009 | Total | 1986 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2008 | Ittoqqortoomiit | 2 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2008 | West and Southeast | 2604 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2008 | Total | 2606 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2007 | Ittoqqortoomiit | 16 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2007 | West and Southeast | 3265 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2007 | Total | 3281 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2006 | Ittoqqortoomiit | 3 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2006 | West and Southeast | 4744 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2006 | Total | 4747 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2005 | Ittoqqortoomiit | 14 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2005 | West and Southeast | 4114 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2005 | Total | 4128 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2004 | Ittoqqortoomiit | 17 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2004 | West and Southeast | 5826 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2004 | Total | 5843 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2003 | Ittoqqortoomiit | 10 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2003 | West and Southeast | 6307 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2003 | Total | 6317 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2002 | Ittoqqortoomiit | 10 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2002 | West and Southeast | 3525 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2002 | Total | 3535 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2001 | Ittoqqortoomiit | 5 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2001 | West and Southeast | 6259 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2001 | Total | 6264 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2000 | Ittoqqortoomiit | 29 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2000 | West and Southeast | 5805 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 2000 | Total | 5834 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 1999 | Total | 7458 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 1998 | Total | 6328 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 1997 | Total | 7500 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 1996 | Total | 9888 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 1995 | Total | 6884 | No quota |
| Greenland | Hooded seal | Cystophora cristata | 1994 | Total | 7959 | No quota |
| Iceland | Hooded seal | Cystophora cristata | 2023 | Iceland | 0 | |
| Iceland | Hooded seal | Cystophora cristata | 2022 | Iceland | 0 | |
| Iceland | Hooded seal | Cystophora cristata | 2021 | Iceland | 0 | |
| Iceland | Hooded seal | Cystophora cristata | 2020 | Iceland | 0 | |
| Iceland | Hooded seal | Cystophora cristata | 2019 | Iceland | 0 | |
| Iceland | Hooded seal | Cystophora cristata | 2018 | Iceland | ||
| Iceland | Hooded seal | Cystophora cristata | 2011-2017 | Iceland | N/A | |
| Iceland | Hooded seal | Cystophora cristata | 2010 | Iceland | 1 | |
| Iceland | Hooded seal | Cystophora cristata | 2004-2009 | Iceland | N/A | |
| Iceland | Hooded seal | Cystophora cristata | 2003 | Iceland | 2 | |
| Iceland | Hooded seal | Cystophora cristata | 2002 | Iceland | 4 (+ 1050 by Norwegians) | |
| Iceland | Hooded seal | Cystophora cristata | 2001 | Iceland | 21 | |
| Iceland | Hooded seal | Cystophora cristata | 2000 | Iceland | 29 | |
| Iceland | Hooded seal | Cystophora cristata | 1992-1999 | Iceland | N/A | |
| Norway | Hooded seal | Cystophora cristata | 2023 | West Ice | 0 | 0 |
| Norway | Hooded seal | Cystophora cristata | 2022 | West Ice | 14 | 0 |
| Norway | Hooded seal | Cystophora cristata | 2021 | Greenland Sea | 16 | 0 |
| Norway | Hooded seal | Cystophora cristata | 2020 | Greenland Sea | 0 | 0 |
| Norway | Hooded seal | Cystophora cristata | 2019 | West Ice | 22 | 0 |
| Norway | Hooded seal | Cystophora cristata | 2018 | West Ice | 17 | 0 |
| Norway | Hooded seal | Cystophora cristata | 2017 | West Ice | 17 | 0 |
| Norway | Hooded seal | Cystophora cristata | 2016 | West Ice | 18 | |
| Norway | Hooded seal | Cystophora cristata | 2015 | West Ice | 11 | |
| Norway | Hooded seal | Cystophora cristata | 2014 | West Ice | 24 | |
| Norway | Hooded seal | Cystophora cristata | 2013 | West Ice | 22 | |
| Norway | Hooded seal | Cystophora cristata | 2012 | West Ice | 21 | |
| Norway | Hooded seal | Cystophora cristata | 2011 | West Ice | 21 | |
| Norway | Hooded seal | Cystophora cristata | 2010 | West Ice | 178 | |
| Norway | Hooded seal | Cystophora cristata | 2009 | West Ice | 413 | |
| Norway | Hooded seal | Cystophora cristata | 2008 | West Ice | 44 | |
| Norway | Hooded seal | Cystophora cristata | 2007 | West Ice | 62 | |
| Norway | Hooded seal | Cystophora cristata | 2006 | West Ice | 3647 | |
| Norway | Hooded seal | Cystophora cristata | 2005 | West Ice | 3826 | |
| Norway | Hooded seal | Cystophora cristata | 2004 | West Ice | 4881 | |
| Norway | Hooded seal | Cystophora cristata | 2003 | West Ice | 5295 | |
| Norway | Hooded seal | Cystophora cristata | 2002 | West Ice | 7191 | |
| Norway | Hooded seal | Cystophora cristata | 2001 | West Ice | 3820 | |
| Norway | Hooded seal | Cystophora cristata | 2000 | West Ice | 1936 | |
| Norway | Hooded seal | Cystophora cristata | 1999 | West Ice | 4446 | |
| Norway | Hooded seal | Cystophora cristata | 1998 | West Ice | 6351 | 5000 |
| Norway | Hooded seal | Cystophora cristata | 1997 | West Ice | 2934 | 9000 |
| Norway | Hooded seal | Cystophora cristata | 1996 | West Ice | 819 | 1700 |
| Norway | Hooded seal | Cystophora cristata | 1995 | West Ice | 933 | 1235 |
| Norway | Hooded seal | Cystophora cristata | 1994 | West Ice | 492 | |
| Norway | Hooded seal | Cystophora cristata | 1993 | West Ice | 384 | |
| Norway | Hooded seal | Cystophora cristata | 1992 | West Ice | 755 | |
This database of reported catches is searchable, meaning you can filter the information by for instance country, species or area. It is also possible to sort it by the different columns, in ascending or descending order, by clicking the column you want to sort by and the associated arrows for the order. By default, 30 entries are shown, but this can be changed in the drop-down menu, where you can decide to show up to 100 entries per page.
Carry-over from previous years are included in the quota numbers, where applicable.
You can find the full catch database with all species here.
For any questions regarding the catch database, please contact the Secretariat at nammco-sec@nammco.no.
BY-CATCH
Very few hooded seals are taken as by-catch in fisheries and this is considered an insignificant source of mortality (Stenson 2006).
CLIMATE CHANGE
Since the hooded seal is so dependent on sea ice for pupping, moulting and resting, they will be greatly affected by any changes that might occur in their sea ice habitats. Sea water temperatures have varied historically, but over the past four decades, there has been a warming trend in the water temperatures throughout the hooded seal’s range. There has also been a decline in sea ice extent and coverage associated with this warming trend since the beginning of the satellite record of ice conditions (1979) (Kovacs et al. 2011, Johnston et al. 2012).
Since 2000, the frequency of light ice years (measured primarily as ice cover) in the Northwest Atlantic whelping areas has increased and the duration of the ice season decreased (Bajzak et al. 2011, Stenson et al. 2016). Hooded seals require areas of thick, usually multi-year sea ice late in the season for successful breeding. The extent of this type of ice cover has been sharply reduced over the last several decades (Kovacs et al. 2011).

© Garry Stenson
Effects
A reduction in the availability or quality of ice for whelping and moulting could affect hooded seal populations in several ways (Kovacs et al. 2011). Pups require ice cover for a few weeks for weaning and as a resting platform as they learn to forage for themselves. If they are forced into the water prematurely, it may reduce their chances of survival because of interrupted nursing, starvation or cold stress. Reduced ice cover might also increase their susceptibility to predation by killer whales and polar bears. These factors may be inhibiting the recovery of the Greenland Sea stock, which continues to decline despite a cessation in hunting (Øigard et al. 2014). There is also some evidence of a reduction in the pregnancy rate among Northwest Atlantic hooded seals, which could also be due to stresses induced by climate change (Frie et al. 2012).
SHIPPING / OIL SPILLS
Another potential threat to hooded seals brought about by climate change is the increasing ship traffic in the Arctic due to reduced ice cover and more open water. The dramatic decline in Arctic sea ice extent over the past few years has raised the possibility of regular cargo traffic being diverted from current shipping routes. Shipping traffic in the Arctic is also predicted to lead to increases in other activities, including resource extraction and tourism (Corbett et al. 2010). Ships could affect hooded seal habitat through their emissions or an accidental fuel spill. They could also affect the ecosystem by introducing invasive species or disrupt the seals with their movements and noise. Oil spills could also lead to direct mortality of hooded seal pups and adults.
CONTAMINANTS
Hooded seals have been found to carry significant loads of contaminants including fluorocarbons (Rotander et al. 2012) and mercury (Dietz et al. 2012). Concentrations of mercury have increased over the past century due to industrialization and atmospheric contamination. Concentrations in hooded seals exceed what are considered safe threshold levels in some areas but the consequences of this are not clear.
FISHING
Reduced availability of potential prey due to fishing activities is a potential threat to hooded seals. Hooded seals feed on many fish species, such as Atlantic cod, redfish, Greenland halibut and blue whiting, which also are valuable commercial species (Folkow and Blix, 1995, 1999, Hauksson and Bogasson 1997, Hammill and Stenson 2000). In some areas, consumption by hooded seals is similar in magnitude to the catch by commercial fisheries. This suggests that competition for these resources could occur (Folkow and Blix 1995, Hammill and Stenson 2000). For example, it is possible that the decline in redfish due to overfishing may be contributing to the continuing decline of the Greenland Sea stock of hooded seals (Kovacs 2016).
NORWAY
In Norway, research on hooded seals is conducted through the Institute of Marine Research (IMR) and the Norwegian Polar Institute (NPI). Some of the research is conducted under the MOSJ programme –Environmental Monitoring of Jan Mayen and Svalbard.
Surveys
The IMR carries out surveys of harp and hooded seals in the Greenland Sea whelping areas at roughly 5 year intervals. The last survey for hooded seals was done in 2018 (National Progress Report Norway 2018, ICES 2019).
Hooded seal surveys use a combination of visual and photographic methods. Visual reconnaissance surveys, using helicopters and fixed wing aircraft, are used to locate and map the whelping patches. Photographic survey transects are then flown over the patches. Pups are counted on the photos by experienced readers and the counts are verified by repeated readings. The pups are classified by “stages” according to their apparent age, from newborn to weaned pup. These data are used in combination with information from tagged pups to estimate the proportion of pups that are on the ice, and not in the water, and thus “available” to be seen, at any time. The corrected pup density on the photographic transects are applied to the entire survey area using standard strip transect methodology.
Estimates of pup production are extended to estimates of the total population using population models. These models incorporate data on pregnancy rate at age, natural mortality and catch at age to calculate the number of adult seals that would produce the number of pups observed in the survey (Hammill and Stenson 2006, Salberg et al. 2008).
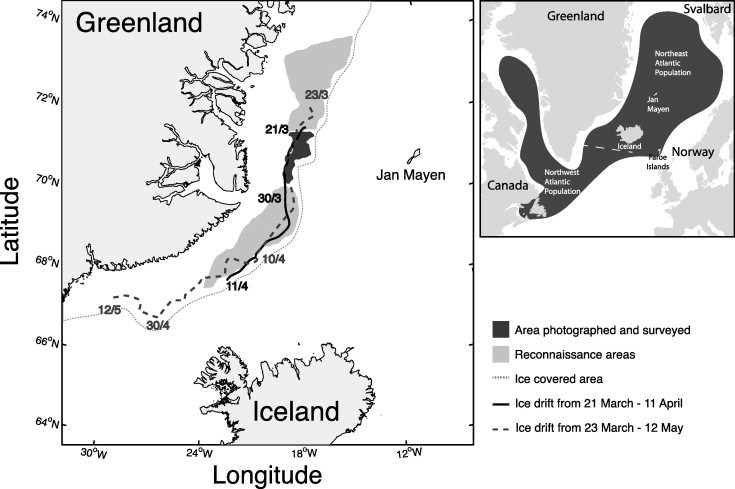
Area covered by photographic survey over seal whelping patches on March 28 and areas covered by reconnaissance flights conducted by air-crafts (22 March-1 April) and helicopter (18 March -1 April). Ice drift in the Greenland Sea during the period 21 March-11 April and 23 March-12 May, as observed from two satellite based GPS beacons deployed on the ice (Oigard et al. 2014).
Evaluation and development of methods
The survey methods used are under continuous evaluation and development. Use of satellite imagery for detection of new whelping areas is being considered, as is the use of drones to replace the fixed-wing aircraft for the photographic surveys (NAMMCO 2014). Inspecting the aerial photos and counting the pups takes much time and effort, and research is ongoing to determine if that process could be automated (ICES 2019). The development of software-based detection methods using artificial intelligence (deep learning) is currently being developed through a collaboration between the Norwegian Computing Centre, the Institute of Marine Research, and Fisheries and Oceans Canada (ICES 2019). Although the results for hooded seals have so far struggled with misclassifications, the use of additional data on this species in the training dataset may improve accuracy.
Satellite tags
Hooded seals are deep-diving animals that range widely through the northern North Atlantic, often through areas covered in pack ice that are inaccessible to most ships. Tagged hooded seals are therefore ideal for obtaining important oceanographic and climate data at relatively low cost.
In 2007 and 2008, 20 hooded seals were instrumented with satellite linked tags. When the seals ascend from a dive, the instrument records vertical profiles of conductivity, temperature and pressure (depth) (CTD). The seals covered an area of more than 3,000,000 km2 in the Greenland and Norwegian seas ranging all the way from south of the Faroe Islands (59ºN) and almost up to the North Pole (88.5ºN). Diving depths were deeper than 1,000 m, and the seals collected more than 7,000 CTD profiles along their tracks. These data have been used to update oceanographic models in an area that is extremely important for climate modelling, showing substantial changes in the area due to climate warming (NAMMCO 2009a, Isachsen et al. 2014).
Ecology, Physiology and Behaviour
In 2019, 6 weaned hooded seal pups were live-captured and transported to Tromsø, where they were maintained in approved sea-water seal tanks at Department of Arctic and Marine Biology, UiT, for use in studies of adaptations to diving and diving-induced hypoxia (National Progress Report Norway 2019).
In 2019, 9 adult and 7 new-born hooded seals were culled by researchers from the Norwegian Institute for Marine Research for use in various collaborative scientific projects. This included research on a) mechanisms underlying neuronal tolerance to lack of oxygen (hypoxia); b) developmental changes in resting metabolism of newborns from birth to weaning to understand how deposition of large fat (blubber) supplies affect their energy expenditure; c) how energy from lactating mothers is deposited into the various internal organs and how such energy deposition changes over time with changing environmental conditions; and d) the patterns of entry and accumulation of mercury (Hg) and how this is affected by feeding behaviour, distribution and key physiological processes such as lactation and fasting (National Progress Report Norway 2019).
There are also efforts underway to develop brain cell cultures for hooded seals to allow for more continuous access to material for detailed cellular studies and reduce the need to cull seals for access to study material (National Progress Report Norway 2019).
GREENLAND
In Greenland, research is conducted by the Greenland Institute of Natural Resources. From 2004-2007, the GINR, in cooperation with Canadian scientists, attached satellite tags to hooded seals in the moulting area off southeast Greenland. These instrumented seals provided a wealth of information on the migration, diving and habitat preferences of hooded seals, as well as oceanographic data from a wide area of the western North Atlantic (Andersen et al. 2013, 2014).
Andersen, J. M., Stenson, G. B., Skern-Maurizen, M., Wiersma, Y. F., Rosing-Asvid, A., Hammill, M. O., and Boehme, L. (2014). Drift diving by hooded seals (Cystophora cristata) in the Northwest Atlantic Ocean. Plos ONE, 9, 1-11. https://doi.org/10.1371/journal.pone.0103072
Andersen, J. M., Skern-Mauritzen, M., Boehme, L., Wiersma, Y. F., Rosing-Asvid, A., Hammill, M. O. and Stenson, G. B. (2013a). Investigating annual diving behaviour by hooded seals (Cystophora cristata) within the Northwest Atlantic Ocean. Plos ONE, 8(11), 1-13. https://doi.org/10.1371/journal.pone.0080438
Andersen, J. M., Wiersma, Y. F., Stenson, G. B., Hammill, M. O., Rosing-Asvid, A., and Skern-Maurizen, M. (2013b). Habitat selection by hooded seals (Cystophora cristata) in the Northwest Atlantic Ocean. ICES Journal of Marine Science, 70(1), 173-185. https://doi.org/10.1093/icesjms/fss133
Bajzak, C. E., Hammill, M. O., Stenson, G. B. and Prinsenberg, S. (2011). Drifting away: implications of changes in ice conditions for a pack-ice-breeding phocid, the harp seal (Pagophilus groenlandicus). Canadian Journal of Zoology, 89, 1050-1062. https://doi.org/10.1139/z11-081
Bowen, W. D., O. T. Oftedal and D. J. Boness. (1985). Birth to weaning in 4 days: Remarkable growth in the hooded seal, Cystophora cristata. Canadian Journal of Zoology, 63, 2841-2846. https://doi.org/10.1139/z85-424
Burns, J. M., Lestyk, K., Folkow, L. P., Hammill, M. O. and Blix, A. S. (2007). Size and distribution of oxygen stores in harp and hooded seals from birth to maturity. Journal of Comparative Physiology B, 177, 687-700. https://doi.org/10.1007/s00360-007-0167-2
Coltman, D. W., Stenson, G., Hammill, M. O., Haug, T., Davis, C. S. and Fulton, T. L. (2007). Panmictic population structure in the hooded seal (Cystophora cristata). Molecular Ecology, 16, 1639-1648. https://doi.org/10.1111/j.1365-294X.2007.03229.x
Corbett, J. J., Lack, D. A., Winebrake, J. J. , Harder, S., Silberman, J. A. and Gold, M. (2010). Arctic shipping emissions inventories and future scenarios. Atmospheric Chemistry and Physics, 10, 9689–9704. https://doi.org/10.5194/acp-10-9689-2010
Department of Fisheries and Oceans (DFO). (2016). 2011-2015 Integrated Fisheries Management Plan for Atlantic Seals. http://www.dfo-mpo.gc.ca/fm-gp/seal-phoque/reports-rapports/mgtplan-planges20112015/mgtplan-planges20112015-eng.htm#c3.1.1
Dietz, R., Sonne, C., Basu, N., Braune, B., O’Hara, T., Letcher, R. J., Scheuhammer, T. et al. (2013). What are the Toxicological Effects of Mercury in Arctic Biota? Science of the Total Environment, 443, 775-790. https://doi.org/10.1016/j.scitotenv.2012.11.046
Folkow, L. P. and Blix, A. S. (1995). Distribution and diving behaviour of hooded seals. In Blix, A. S., Walløe, L. and Ulltang, Ø. (eds.), Whales, seals, fish and man (pp. 193-202). Amsterdam: Elsevier. https://doi.org/10.1016/S0163-6995(06)80023-5
Folkow, L. P. and Blix, A. S. (1999). Diving behaviour of hooded seals (Cystophora cristata) in the Greenland and Norwegian seas. Polar Biology, 22, 61-74. https://doi.org/10.1007/s003000050391
Folkow, L. P., Ramirez, J., Ludvigsen, S., Ramirez, N. and Blix, A. S. (2008). Remarkable neuronal hypoxia tolerance in the deep-diving adult hooded seal (cystophora cristata). Neuroscience Letters, 446, 147-150. https://doi.org/10.1016/j.neulet.2008.09.040
Folkow, L., Mårtensson, P. and Blix, A. (1996). Annual distribution of hooded seals (Cystophora cristata) in the Greenland and Norwegian seas. Polar Biology, 16, 179-189. https://doi.org/10.1007/BF02329206
Folkow, L., Nordøy, E. and Blix, A. (2010). Remarkable development of diving performance and migrations of hooded seals (Cystophora cristata) during their first year of life. Polar Biology, 33(4), 433-441. https://doi.org/10.1007/s00300-009-0718-y
Frie, A. K., Stenson, G. B. and Haug, T. (2012). Long-term trends in reproductive and demographic parameters of female Northwest Atlantic hooded seals (Cystophora cristata): population responses to ecosystem change? Canadian Journal of Zoology, 90, 376-392. https://doi.org/10.1139/z11-140
Hammill, M. O. and Stenson, G. B. (2000). Estimated prey consumption by harp seals (Phoca groenlandica), hooded seals (Cystophora cristata), grey seals (Halichoerus grypus) and harbour seals (Phoca vitulina) in Atlantic Canada. Journal of Northwest Atlantic Fishery Science, 26, 1-23. https://doi.org/10.2960/J.v26.a1
Hammill, M. O. and Stenson, G. B. (2006). Abundance of Northwest Atlantic hooded seals (1960 – 2005). DFO Canadian Science Advisory Secretariat. Res. Doc. 2006/068. http://www.dfo-mpo.gc.ca/csas-sccs/publications/resdocs-docrech/2006/2006_068-eng.htm
Hammill, M. O. and Stenson, G. B. (2007). Application of the precautionary approach and conservation reference points to management of Atlantic seals. ICES Journal Of Marine Science, 64, 702-706. https://doi.org/10.1093/icesjms/fsm037
Harris, D. E., Lelli, B., Jakush, G. and Early, G. (2001). Hooded seal (Cystophora cristata) records from the southern Gulf of Maine. Northeastern Naturalist, 8, 427-434. https://doi.org/10.1656/1092-6194(2001)008[0427:HSCCRF]2.0.CO;2
Haug, T., Nilssen, K. T., and Lindblom, L. (2004). Feeding habits of harp and hooded seals in drift ice waters along the east coast of Greenland in summer and winter. Polar Research, 23, 35-42. https://doi.org/10.3402/polar.v23i1.6264
Haug, T., Nilssen, K. T., Lindblom, L., and Lindstrøm, U. (2007). Diets of hooded seals (Cystophora cristata) in coastal waters and drift ice waters along the east coast of Greenland. Marine Biology Research, 3, 123-133. https://doi.org/10.1080/17451000701358531
Hauksson, E., and Bogason, V. (1997). Comparative feeding of grey (Halichoerus grypus) and common seals (Phoca vitulina) in coastal waters of Iceland, with a note on the diet of hooded (Cystophora cristata) and harp seals (Phoca groenlandica). Journal of Northwest Atlantic Fishery Science, 22, 125-135. https://doi.org/10.2960/J.v22.a11
Hayssen, V. (1995). Milk: It does a baby good. Natural History, 104, 36.
International Council for the Exploration of the Sea (ICES). (2016). Report of the ICES/NAFO/NAMMCO Working Group on Harp and Hooded Seals (WGHARP), 26-30 September 2016, ICES HQ, Copenhagen, Denmark. ICES CM 2016/ACOM:21. 85 pp. Available at: http://nammco.wpengine.com/wp-content/uploads/2017/01/ices-nammco-nafo-wgharp-report-final.pdf
International Council for the Exploration of the Sea (ICES). (2019). ICES/NAFO/NAMMCO Working Group on Harp and Hooded Seals (WGHARP). ICES Scientific Reports, 1, 72. 193pp. http://www.ices.dk/sites/pub/Publication%20Reports/Forms/DispForm.aspx?ID=36286
Isachsen, P. E., Sørlie, S. R., Mauritzen, C., Lydersen, C., Dodd, P. and Kovacs, K. M. (2014). Upper-ocean hydrography of the Nordic Seas during the International Polar Year (2007–2008) as observed by instrumented seals and Argo floats. Deep Sea Research, 93, 41-59. https://doi.org/10.1016/j.dsr.2014.06.012
Johnston, D. W., Bowers, M. T., Friedlaender, A. S. and Lavigne, D. M. (2012). The Effects of Climate Change on Harp Seals (Pagophilus groenlandicus). PLoS ONE , 7(1), e29158. https://doi.org/10.1371/journal.pone.0029158
Kapel, F. O. (1995). Feeding ecology of harp and hooded seals in the Davis Strait—Baffin Bay region. Developments in Marine Biology, 4, 287-304. https://doi.org/10.1016/S0163-6995(06)80031-4
Kovacs, K. M. (2002). Hooded seal Cystophora cristata. In W. F. Perrin, B. Wursig and J. G. M. Thewissen (eds.), Encyclopedia of Marine Mammals (pp. 580-583). San Diego: Academic Press.
Kovacs, K. M., Lydersen, C., Hammill, M. O. and Lavigne, D. M. (1996). Reproductive effort of male hooded seals (Cystophora cristata). Canadian Journal of Zoology, 74, 1521-1530. https://doi.org/10.1139/z96-166
Kovacs, K. M., Lydersen, C., Overland, J. and Moore, S. (2011). Impacts of changing sea-ice conditions on arctic marine mammals. Marine Biodiversity, 41, 181-194. https://doi.org/10.1007/s12526-010-0061-0
Kovacs, K. M. (2016). Cystophora cristata. The IUCN Red List of Threatened Species 2016: e.T6204A45225150. http://dx.doi.org/10.2305/IUCN.UK.2016-1.RLTS.T6204A45225150.en
Kovacs, K. M. and Lavigne, D. M. (1986). Cystopora cristata. Mammalian Species, 258, 1-9. https://doi.org/10.2307/3503888
Kovacs, K. M. and Lavigne, D. M. (1992). Mass transfer efficiency between hooded seal (Cystophora cristata) mothers and their pups in the Gulf of St. Lawrence. Canadian Journal of Zoology, 70, 1315-1320. https://doi.org/10.1139/z92-184
Lavigne, D. M. and Kovacs, K. M. (1988). Harps and hoods: ice-breeding seals of the northwest Atlantic. Ontario: University of Waterloo Press.
Leclerc, L., Lydersen, C., Haug, T., Bachmann, L., Fisk, A. and Kovacs, K. (2012). A missing piece in the Arctic food web puzzle? stomach contents of Greenland sharks sampled in Svalbard, Norway. Polar Biology, 35, 1197-1208. https://doi.org/10.1007/s00300-012-1166-7
Lydersen, C. and Kovacs, K. M. (1999). Behaviour and energetics of ice-breeding, North Atlantic phocid seals during the lactation period. Marine Ecology Progress Series, 187, 265-281. https://doi.org/10.3354/meps187265
Ministry of Fisheries, Hunting and Agriculture (MFHA), Greenland. (2012). Management and utilization of seals in Greenland. http://portugal.um.dk/en/about-denmark/greenland-and-the-faroe-islands/greenland//~/media/Portugal/Documents/White%20paper%20on%20seals%20in%20Greenland_May2012_eng.pdf
Mignucci-Giannoni, A. A. and Haddow, P. (2002). Wandering hooded seals. Science, 295, 627-628. https://doi.org/10.1126/science.295.5555.627
North Atlantic Marine Mammal Commission (NAMMCO). (2004a). Report of the 12th Meeting of the Scientific Committee. In NAMMCO Annual Report 2004. NAMMCO, Tromsø, Norway, pp. 207-280. Available at https://nammco.no/topics/annual-reports/
North Atlantic Marine Mammal Commission (NAMMCO). (2004b). Report of the NAMMCO Workshop on Hunting Methods for Seals and Walrus. https://nammco.no/assets/Uploads/Report-from-the-Workshop-on-Hunting-Methods-for-Seals-and-Walrus-2004.pdf
North Atlantic Marine Mammal Commission (NAMMCO). (2005). Report of the 13th Meeting of the Scientific Committee. In NAMMCO Annual Report 2005. NAMMCO, Tromsø, Norway. pp. 161-310. Available at https://nammco.no/topics/annual-reports/
North Atlantic Marine Mammal Commission (NAMMCO). (2006a). Report of the 14th Meeting of the Scientific Committee. In NAMMCO Annual Report 2006 – Volume II. NAMMCO, Tromsø, Norway, pp. 287-488. Available at https://nammco.no/topics/annual-reports/
North Atlantic Marine Mammal Commission (NAMMCO). (2006b). Report of the NAMMCO workshop to address the problems of “struck and lost” in seal, walrus and whale hunting. Available at https://nammco.no/assets/Publications/Hunting-Methods-Committee/Report-of-WS-on-Struck-and-Lost-November-2006.pdf
North Atlantic Marine Mammal Commission (NAMMCO). (2009a). Report of the 16th Meeting of the Scientific Committee. In NAMMCO Annual Report 2009. North Atlantic Marine Mammal Commission, Tromsø, Norway, pp. 237-456. Available at https://nammco.no/topics/annual-reports/
North Atlantic Marine Mammal Commission (NAMMCO). (2009b). Report of the NAMMCO Expert Group Meeting On Best Practices in the Hunting and Killing of Seals. Available at https://nammco.no/assets/Publications/Hunting-Methods-Committee/NAMMCO-Report-Expert-Group-on-Best-Practises-in-Hunting-and-Killing-of-Seals.pdf
North Atlantic Marine Mammal Commission (NAMMCO). (2012). Report of the 17th Meeting of the Scientific Committee. In NAMMCO Annual Report 2012. NAMMCO, Tromsø, Norway, pp. 263-550. Available at https://nammco.no/topics/annual-reports/
North Atlantic Marine Mammal Commission (NAMMCO). (2013). Report of the 20th Meeting of the Scientific Committee. In NAMMCO Annual Report 2013. NAMMCO, Tromsø, Norway, pp. 153-240. Available at https://nammco.no/topics/annual-reports/
North Atlantic Marine Mammal Commission (NAMMCO). (2014). Report of the 21st Meeting of the Scientific Committee. In NAMMCO Annual Report 2014. NAMMCO, Tromsø, Norway, pp. 125-190. Available at https://nammco.no/topics/annual-reports/
Rotander, A., Kärrman, A., Bavel, B. v., Polder, A., Rigét, F., Auðunsson, G. A., … and Dam, M. (2012). Increasing levels of long-chain perfluorocarboxylic acids (PFCAs) in Arctic and North Atlantic marine mammals, 1984–2009. Chemosphere, 86, 278-285. https://doi.org/10.1016/j.chemosphere.2011.09.054
Salberg, A., Haug, T., and Nilssen, K. T. (2008). Estimation of hooded seal (Cystophora cristata) pup production in the Greenland sea pack ice during the 2005 whelping season. Polar Biology, 31, 867-878. https://doi.org/10.1007/s00300-008-0425-0
Sergeant, D. E. (1976). History and present status of populations of harp and hooded seals. Biological Conservation, 10, 95-118. https://doi.org/10.1016/0006-3207(76)90055-0
Sergeant, D. E. (1991). Harp seals, man and ice. Canadian Special Publication on Fisheries and Aquatic Sciences, 114, 153 p. https://www.arlis.org/docs/vol1/K/24728024.pdf
Sergeant, D. E. (1974). A rediscovered whelping population of hooded seals Cystophora cristata Erxleben, and its possible relationship with other populations. Polarforschung, 44, 1-7. https://doi.org/10.2312/polarforschung.44.1.1
Stenson, G. B. (2006). Hunt induced mortality in Northwest Atlantic Hooded Seals. DFO Canadian Science Advisory Secretariat. Res. Doc. 2006/066. http://www.dfo-mpo.gc.ca/csas-sccs/publications/resdocs-docrech/2006/2006_066-eng.htm
Stenson, G.B., Buren, A.D. and Koen-Alonso, M. (2016). The impact of changing climate and abundance on reproduction in an ice-dependent species, the northwest Atlantic harp seal, Pagophilus groenlandicus. ICES Journal of Marine Science, 73, 250-262. https://doi.org/10.1093/icesjms/fsv202
Stenson, G.B., Ni, I.H., Ross, S.A. and McKinnon, D. (1991). Hooded seal, Cystophora cristata, feeding and interactions with commercial fisheries in Newfoundland. DFO Canadian Science Advisory Secretariat. Res. Doc. 1991/45. http://www.dfo-mpo.gc.ca/csas-sccs/publications/resdocs-docrech/1991/1991_045-eng.html
Thordarson, G., Vikingsson, G. A. and Hersteinsson, P. (2007). Seasonal variation in body condition of adult male hooded seals (Cystophora cristata) in Skjalfandi-bay, northeast Iceland. Polar Biology, 30, 379-386. https://doi.org/10.1007/s00300-006-0194-6
Øigård, T. A., Haug, T. and Nilssen, K. T. (2014). Current status of hooded seals in the Greenland sea. Victims of climate change and predation? Biological Conservation, 172, 29-36. https://doi.org/10.1016/j.biocon.2014.02.007