Harbour Seal
Latest update: August 2024
The harbour seal (Phoca vitulina) is a small phocid seal. It has the broadest geographical distribution range of all seal species, ranging from temperate to polar coastal waters of the Northern Hemisphere. The world’s northernmost harbour seal population occurs at Prins Karls Forland on the west coast of Svalbard. This isolated population is protected and numbers almost 2,000 animals.
Adults average around 150 cm in length and 70-100 kg in weight, and the males are slightly larger than the females. Their coat colour pattern is very variable, but is usually silvery and darker on the back, creamy or lighter-grey on the belly and flanks, with dark spots covering the entire body. Pups are usually born in their adult-pattern coat.
Harbour seals are generalist predators but prefer small to medium-sized fish. They are a coastal species that can be found hauling out in groups of up to several hundred animals.
ABUNDANCE
There are estimated to be approximately 200,000 harbour seals in the North Atlantic.
DISTRIBUTION
In the North Atlantic, they inhabit temperate to polar waters along the eastern and western coasts. Svalbard is home to the northernmost population of harbour seals.
RELATION TO HUMANS
Harbour seals are hunted in Norway and Iceland for their meat and skin.
CONSERVATION AND MANAGEMENT
Management advice for harbour seals in the North Atlantic is provided by NAMMCO. Management is the responsibility of each country.
In the most recent assessments, the species is listed as being of ‘Least Concern’ on the global (2016) and the European (2023) IUCN Red Lists as well as on the 2021 Norwegian red list. However, it is listed as ‘Near threatened’ on the 2021 Svalbard (Norway) red list, as ‘Critically Endangered’ on the 2018 Greenlandic red list , and as ‘Endangered’ on the 2021 Icelandic red list. Harbour seals are protected in Svalbard and Greenland.
Lifespan
Females: 30-35 years, Males: 20-25 years.
Productivity
One pup per year.
Feeding
Generalist predator, with an overall preference for small to medium sized fish including cod- and flatfishes, herring, sculpins and sandeels.
Size
Around 150 cm in length and 70–100 kg in weight, with males being somewhat larger than females.
General characteristics
The harbour seal is a coastal, non-migratory species. It is the most widely distributed pinniped species in coastal areas. It is found across temperate to sub-Arctic waters along the eastern and western coasts of the North Atlantic and the North Pacific Oceans (Bjørge et al. 2010), with some populations also present in the high Arctic (Svalbard and Baffin Island).
Harbour seals are small phocid seals (also called “true” or “earless” seals). They average around 150 cm in length and 70–100 kg in weight, males are generally larger than females. Individuals from the Svalbard population exhibit a high degree of size dimorphism (difference) between sexes compared to many other populations. Males in this population are longer and heavier than females, which might be beneficial during the mating period when males hold underwater territories for long periods of time (VanParijs et al. 1997, Lydersen and Kovacs 2005).
The colour pattern of their coat is very variable, but is usually silvery and darker on the back, creamy or lighter-grey on the belly and flanks, with dark spots covering the entire body. Some individuals have light rings on their back and sides (Fig. 2), which can make them look alike ringed seals (Pusa hispida). They have a compact body with short front and rear flippers.
Harbour seals have a rounded head and a muzzle of moderate length with a concave facial profile. This concave profile makes adults very easily identifiable from adult grey seals, which have a “Roman nose”. Harbour seals’ noses create a dog-like snout with V-shaped nostrils.
Three subspecies are currently recognised (Teilman and Galatius 2018):
- the Atlantic harbour seal (P. v. vitulina; Linnaeus 1758), the only subspecies found in the NAMMCO area
- the Pacific harbour seal (P. v.richardii; Gray 1864)
- the Ungava or Lac des Loups Marins harbour seal (P. v. mellonae, Doutt 1942), a subspecies landlocked and endemic to the freshwater lake system draining into Hudson Bay on the Ungava Peninsula in Northern Quebec. The Ungava harbour seal is considered endangered by the Committee on the Status of Endangered Wildlife in Canada, COSEWIC (Enns et al. 2020).

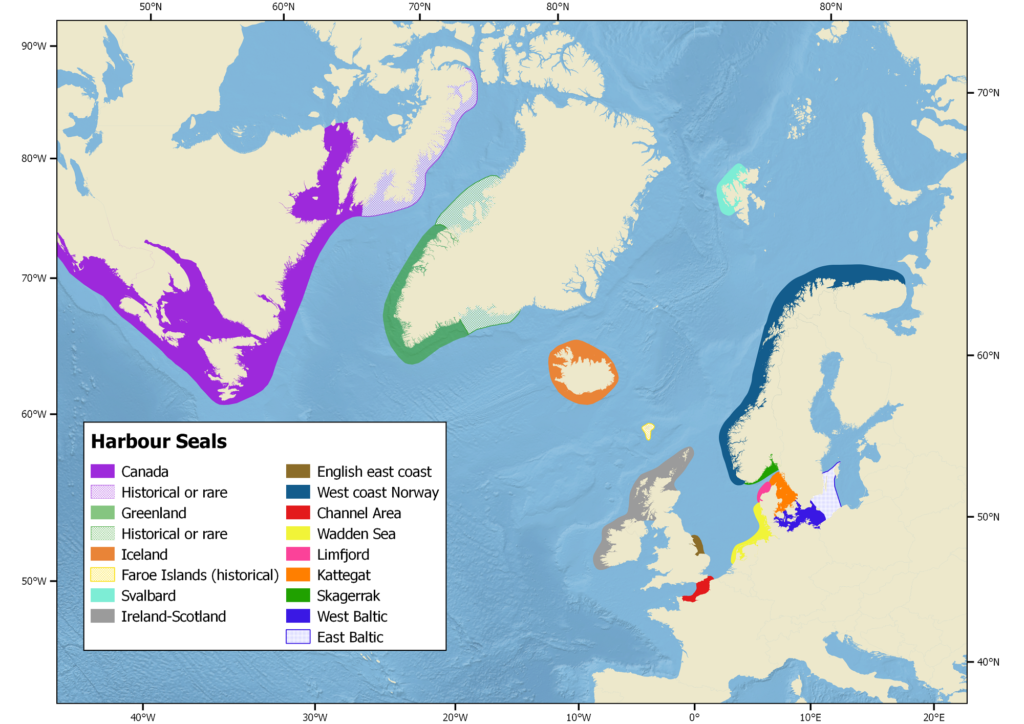
Distribution of harbour seals (Blanchet et al. 2021). Colours correspond to each subspecies. Blue: P. vitulina vitulina; green: vitulina richardii; red: P. vitulina mellonae.
The populations in the Atlantic and the Pacific are completely isolated from each other by the central Canadian High Arctic and the Russian north coast where harbour seals have never been reported (Teilmann and Galatius 2018).
Behaviour

© Icelandic Seal Centre
Harbour seals typically use solid substrates to haul out, moult, give birth and nurse their young. This includes on beaches, sandbanks and rocks, usually in the intertidal zone along the coast. Although harbour seals may undertake long distance foraging trips lasting several days to weeks, they often show strong site fidelity to the location where they were born during the breeding and moulting seasons (from mid-June to early September) (Tougaard et al. 2008, Dietz et al. 2013, Blanchet et al. 2014). They move between haul-out sites and feeding areas, but rarely show seasonal migration. There are, however, deviations from these rules and a tracking study in southeast Greenland showed seals from distant breeding locations (250 km apart) using the same moulting haul-out site (Rosing-Asvid et al. 2020). In another example, harbour seals from the St. Lawrence River estuary, Canada have shown seasonal migrations related to ice cover, with a tagged seal from this estuary holding the record tracking distance (520 km) away from its tagging site (Lesage et al. 2004).
Harbour seals are opportunistic feeders, foraging mostly in coastal areas on locally abundant and easily available prey items, primarily. They consume more benthic (on the bottom of the sea) than pelagic (in the water column) species. Depending on prey type, they use different underwater feeding techniques, including ambush, active search or digging with final pursuit (Bowen et al. 2002).
Diving and Swimming Behaviour
Harbour seals dive to depths in excess of 400 m at several locations, the deepest recorded dive being 631m off southeast Greenland (Gjertz et al. 2001, Rosing-Asvid et al. 2020). Dive depths are, however, strongly related to the bathymetry and on average, harbour seals typically perform much shallower dives (60-100 m), mainly as a consequence of their habitat choice (Sharples et al. 2012, Blanchet et al. 2014, Ramasco 2015). Harbour seals also perform resting dives, similar to the typical drift dives performed by other phocid seals (Crocker et al. 1997, Ramasco et al. 2013). Harbour seals exhibit strong seasonal variation in their diving behaviour, with dives being deeper, longer, less numerous and more pelagic during the winter/early spring compared to the fall (Blanchet et al. 2015). Although harbour seals may undertake long-distance foraging trips, the range, depth and duration of movements decreases substantially during the mating season (e.g., Tollit et al. 1998, Bjørge et al. 2002, Dietz et al. 2012, Rosing-Asvid et al. 2020).
As many other seals, harbour seals often swim upside down. Their eyes are oriented toward the top of their head and unless they are diving very deep, swimming upside down gives them a better view of their surroundings.
Social and Hauling–out Behaviour
They are not particularly social animals. Although they gather to rest at haul out sites on a regular basis, they rarely touch each other. In water they mostly feed independently and are seen alone or in small groups.
Haul–out numbers peak during breeding (in the North Atlantic typically 3–4 weeks during May–July) and moulting (in the North Atlantic typically 4–5 weeks during August–September). Generally, seals spend most of their time hauled out during daytime and low tide, while most foraging trips take place at night (Bjørge et al. 1995, Cronin et al. 2009, Ramasco et al. 2014). A tracking study from southeast Greenland showed that during August–September (the molt) on average, the seals hauled-out 7–9 h/day. During November–December there was a gradual transition toward the January-May haul-out mode, with an average of about 4 hours of haul-out every second day (Rosing-Asvid et al. 2020).
Harbour seal haul-out patterns are also directly influenced by water and air temperature and wind. Low tides during warm, calm weather produce the largest numbers of hauled out harbour seals (Granquist and Hauksson 2016). In colder environments, harbour seals must thermoregulate to mitigate heat loss at sea, on land and or ice, which is costly. In Svalbard, at the northern limit of their distributional range, harbour seals typically spend more time at sea during stormy weather conditions, even if the temperature is high because of the wind chill effect (Hamilton et al. 2014). At the southern limit of their distributional range, harbour seals face the opposite problem with hyperthermia observed in juvenile seals at an ambient air temperature of 35 °C (Blanchet et al. 2021).
Acoustic and Breeding Behaviour
Harbour seals are not very vocal but do utilise non-harmonic vocalizations to maintain breeding territories, attract mates during specified times of year, and during mother and pup interactions. Bjørge and Nilssen (Institute of Marine Research 2020) describe the vocalisation mating behaviour as follows: The largest and most dominant males establish territories on the best sites through fighting, likely the sites closest to areas where females haul out with pups. When the females enter oestrus, the males spend a lot of time in their display territories. They dive to a few meters and “sing” two to three times per dive. They continue this behaviour for hours, day after day. Females choose males for mating according to the quality of their “song” and prefer large dominant males, which “sing” with the deepest voice (lowest frequency) and longest duration. Males mate with as many females as they manage to attract.
Life History and Ecology
Reproduction
Females become sexually mature at age 4–5 years and males at age 4–6 years. In the eastern Atlantic, the maximum recorded ages are 36 years for females and 31 years for males. The longevity of Svalbard harbour seals is short compared to other regions, with almost no animal older than 16 years. This is surprising given the limited negative human-seal interactions and the absence of mortality from epizootic outbreak for this population (Lydersen and Kovacs 2005).
The annual reproductive cycle of most seal species is characterized by a tight synchrony of births, ensuring that pups are born at the optimal time of year (Boyd 1991). Across their range, harbour seals give birth to one pup per year between February and July, depending on the environmental conditions and population status, with lower latitudes and populations in good conditions breeding earlier (Reijnders et al. 2010).
Harbour seal pregnancies last around nine months. Pups can occasionally carry a fluffy white coat at birth, but this lanugo is usually shed in the uterus and pups are born in their adult-pattern coat (Bjørge et al. 2010). Harbour seals give birth on platforms (sandy beaches, intertidal rocks, or glacial ice) that can be unstable or flooded rapidly and pups need to be extremely precocial at birth. The pup, which weighs 11–16 kg, is able to swim and dive already just a few minutes after birth and is active at sea during the entire nursing period.
Lactation lasts about three to four weeks, followed by a post-weaning fast that lasts approximately 15 days (Muelbert and Bowen 1993). Male harbour seals come into breeding areas towards the end of the lactation time and perform underwater displays to attract females to mate again (Van Parijs et al. 1997). Although harbour seals mate during this period, the females have delayed implantation (the embryo does not implant during the immediate period following fertilisation but remains in a state of suspended growth).
Feeding
Foraging has priority from mid-September to mid-June. During this period, harbour seals will disperse to sites with good food availability, often several tens of kilometres away from the breeding and moulting sites. Harbour seals are generalist predators, with an overall preference for small to medium sized fish (10–20 cm). They commonly feed on codfishes (Gadidae), sandeels (Ammodytes sp.), sculpins (Cottidae), herring (Clupea harengus), capelin (Mallotus villosus) and flatfishes (Pleuronectidae) depending on their surrounding habitat (Härkönen 1987, Olsen and Bjørge 1995, Bowen and Harrison 1996, Tollit et al. 1998, Colominas 2012, Ramasco 2015, Wilson and Hammond, 2016, Granquist et al. 2018, Sørlie et al. 2020).
Geographical, seasonal and interannual variability in their diet) is documented across their geographical range (Härkönen 1987, Pierce et al. 1991, Hall et al. 1998, Spitz et al. 2010, Wilson and Hammond 2016). The shifts in diet over time are often linked to changes in the most abundant prey species to be found in their preferred size group, and harbour seals respond to seasonal prey pulses, such as seasonal increased concentration of herring or salmon (Colominas 2012, Middlemas et al. 2006, Thomas et al. 2011, Wilson and Hammond 2016, Ramasco et al. 2017).
Predators and interspecific competition
In the North Atlantic, harbour seal predators include killer whale (Orcinus orca) sharks, eagles, ravens, gulls, grey seals (Halichoerus grypus), polar bear (Ursus maritimus), walrus and shore-based predators such as the red fox (Vulpes vulpes) and Arctic fox (Alopex lagopus), wolf (Canis lupus), and brown bear (U. Arctos). Sharks are thought to have heavily impacted harbour seals population demography on Sable Island off Nova Scotia (Lucas and Stobo 2000) and might also do so off Svalbard (Leclerc et al. 2012).
In Norway, during winter, killer whales follow the over-wintering herring and may move closer to seal colonies. They have certainly been reported to eat harbour and grey seals. In Scotland, killer whale predation on harbour seals seems to be on the increase. The predation pressure of polar bears may also increase as they spend more time on land and ringed seals may become less abundant (see also under climate change).
Grey seals may also be a source of mortality for harbour seals through competition and or direct predation. Harbour seals appear to be declining in many areas where grey seals are increasing (NAMMCO 2016. Although there is still little data to assess whether grey seals could have a population impact on harbour seals (ICES 2017), they may have a detrimental effect on the abundance of harbour seals through competition and or direct predation as reported in Sable Island (Canada), New England (USA), the Baltic Sea and Scotland (Bowen et al. 2003, van Neer et al. 2015, NAMMCO 2016, SCOS 2018, Wilson and Hammond 2019). Female harbour seals have been reported dying after coercive copulation by male grey seals in the Wadden Sea (Rohner et al. 2020).
Phocine Distemper Virus
Harbour seal abundances have fluctuated widely in the Northeast Atlantic in recent decades, mainly due to local outbreaks of the Phocine Distemper Virus (PDV). A first epidemic in 1988 killed about 50% of the harbour seals in Skagerrak-Kattegat, the Wadden Sea and the Wash in the UK, with the subsequent very rapid recovery of about 13% increase per year, and even higher growth rates observed regionally (Härkönen et al. 2006, Reijnders et al. 2010, Olsen et al. 2010). A new PDV epizootic hit the same populations in 2002 with mortality similar to (or in some areas less than) in 1988. Animals that gained immunity during the first epizootic may still have been part of the population in 2002 and thus contributed to limiting the mortality.
Harbour seal populations along the Norwegian coast escaped both epidemics, due either to genetic difference or immunity, the spread being limited to southern Norway and the Skagerrak. The populations north of 65 °N have not been affected by these recurrent epidemics. However, if grey seals expand their distributional range northwards, they may come in contact with immunologically naive northern populations of harbour seals such as in Greenland (Rosing-Asvid et al. 2010). The PDV epidemics are thought to have started through contact between harbour seals and grey and harp seals. Harp seals from the eastern Arctic were the source and reservoir of infection and grey seals, which show long-distance movements, acted as sub-clinical infected carriers contributing to the spread among regions and the sympatric colonies of the more philopatric harbour seals (Härkonen et al. 2006, Härkönen and Harding 2010, Duignan et al. 2014).
Distribution and Habitat
Only present in the Northern hemisphere, the harbour seal is the world’s most widely distributed pinniped species ranging from temperate to Arctic regions along the eastern and western coasts of the North Atlantic (from 30 to 78.5° N) and the North Pacific (from 28 to 61.2° N). In the east Atlantic, these species range from northern France (30°N) to Svalbard (78.5°N) and over Iceland and Greenland in the central Atlantic. Along the western Atlantic coast, the harbour seal ranges from New Jersey (40°N) in the United States, north to Baffin Island (73°N) in Canada, including Hudson Bay and Foxe Basin (Teilmann and Galatius 2018). The Ungava harbour seal is a freshwater subspecies endemic to the lake system draining into Hudson Bay on the Ungava Peninsula in Northern Quebec.
Harbour seals use an array of habitats including bays, rivers, lakes, estuaries, intertidal habitats, sea ice, and icebergs in tidewater glacier fjords (Vincent et al. 2010, Merkel et al. 2013, Rosing-Asvid et al. 2020). Harbour seals are central-place foragers, regularly hauling out on land in between spending time at sea travelling and foraging and their at-sea distribution is strongly linked to their haul out locations (Jones et al. 2017). They primarily use areas close to the coastline, within 30–50 km, animals spending the majority of time within a few kilometres of the coast in shallow water of less than 50m (Tollit et al. 1998, Sharples et al. 2009, Jones et al. 2015, 2017). In some areas, harbour seals are seen in freshwater rivers and up to lakes.
Given its broad geographic distribution, the species encounters an extensive gradient of environmental conditions from temperate to Arctic. The species thus provides a unique opportunity for understanding the influence of changing environmental conditions on a single species (Blanchet et al. 2021).
North Atlantic stocks
Andersen and Olsen (2010) reviewed genetic studies on harbour seal populations in the North Atlantic and identified twelve distinct populations: USA/Canada, Iceland, west coast of Norway, Ireland-Scotland, English east coast, Channel area, Wadden Sea, Limfjord, Skagerrak, Kattegat, West Baltic, and East Baltic/Baltic proper. Population structure was mostly addressed at the regional level and not at the local level, i.e., within countries and Andersen and Olsen underlined it is underlined that, where number of genetically distinct populations could well be higher than 14.
A subsequent study investigated the genetic status of the Svalbard population in the context of nearby populations in Iceland, south‐east Greenland, and northern Norway, and found them to all be distinct (Andersen et al. 2011). The low genetic diversity in the Svalbard population indicated possible signs of inbreeding. The population located in southeast of Greenland were genetically close to the Icelandic and Svalbard populations. Surprisingly, despite low numbers, the population in southeast Greenland exhibited a high level of genetic diversity, as high as in the much larger population in Europe, indicating supplies of genes from Iceland and Svalbard, which might explain its better survival.
The first finer spatial scale study of harbour seals population structure in the UK and neighbouring localities on the European mainland (Olsen et al. 2017) suggested an initial division into two main groups consisting of localities in northern UK and southern UK–mainland Europe. The northern cluster was further divided into a north-western cluster and north-eastern cluster. The southern cluster was further split into a south‐eastern UK cluster including sites in France and the Dutch Wadden Sea, whereas the Norwegian harbour seals appeared to form a separate fourth cluster. These four major genetic clusters each showed indications of further genetic structuring.
The recent genetic evidence of fine scale population structure in different areas (UK, Olsen et al. 2017; Danish and Swedish waters, Olsen et al. 2014; the northern Japanese island of Hokkaido, Mizuno et al. 2020), combined with information gained from the movement pattens of harbour seals in Norway and Greenland (Bjørge et al. 2002b, Ramasco 2015, Rosing-Asvid et al. 2020), underline the need for finer scale population structure. The current recent telemetry study suggests, for example, that the harbour seals in southern Greenland could consist of two distant and separated breeding populations that share the same foraging and moulting area outside the breeding season (Rosing-Asvid et al. 2020). Harbour seals show strong site fidelity, in particular in the summer season when they are counted. Using large non-biological administrative management units (like in Norway) increases the risk that small, local, genetic genuine populations be wiped out.
Current Abundance and Trends
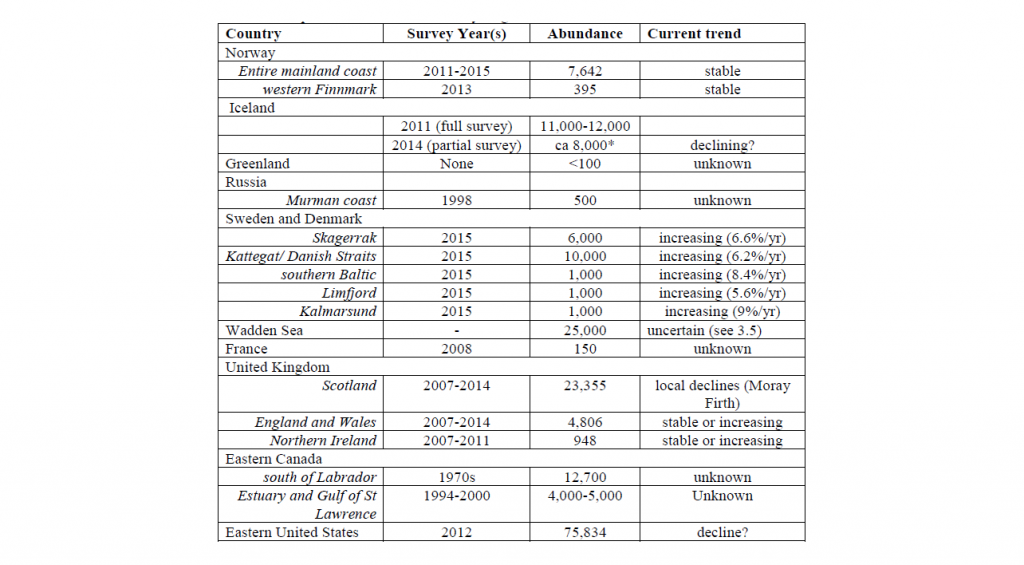
The world population of harbour seals, including the Atlantic and Pacific stocks, has been estimated at 610,000 – 640,000 individuals (Bjørge et al. 2010). In 2016, the NAMMCO Scientific Committee Coastal Seals Working Group (NAMMCO 2016) reviewed and summarised abundance estimates and trends in the North Atlantic as follows.
New abundance estimates have become available since this 2016 review. Survey counts in 2016–2021 resulted in an estimated total minimum of 6,857 harbour seal in mainland Norway (NAMMCO 2023). The population in Svalbard was estimated to be around 1800 seals in 2009 and 2010 (Merkel et al. 2013). In Iceland, the harbour population was estimated to 9,434 seals (95% CI: 6,149–12,726) in 2018 and to 10,319 seals (95% CI: 6,733–13,906) in 2020 (Granquist and Hauksson 2022).
Status of Stocks within the remit of NAMMCO management
The species is listed as “Least Concern” on the 2016 global IUCN Red List, as it is very widely distributed and the total population size numbers in the 600,000 (Lowry 2016). It is listed also listed as “Least Concern” in 2023 on the European IUCN Red List. However, given its broad geographic distribution, dramatic differences exist between subspecies, regions or populations in terms of minimum population estimates and population dynamics. Both in the North Pacific and the North Atlantic, some populations are stable or increasing whereas others are experiencing declines leading to conservation concerns. This status review focuses on the stocks within the remit of NAMMCO management advice. Blanchet et al. (2021) provides the latest overall review of all harbour seal populations. Regular status updates on North Atlantic harbour seal stocks can be found in the reports of the NAMMCO Scientific Committee Working Group on Coastal Seals.
The NAMMCO Council has requested the regular assessment of the species in its management area. As a result, the Scientific Committee has set up a permanent Working Group, the Working Group on Coastal Seals, to review the status of harbour seals throughout the North Atlantic. The Working Group assessed the status of harbour seals for the first time in 2006, which resulted in Volume 8 of the NAMMCO Scientific Publications series being published in 2010 with a focus on harbour seals in the North Atlantic and Baltic. The working group met again in 2016 and noted that harbour seals appear to be declining in many areas. This was especially in areas where grey seals are increasing, which may have a detrimental effect on the abundance of harbour seals through competition and or direct predation (see Other Source of Mortality – Predation for more details).
Greenland
The harbour seal is distributed along the section of the Greenlandic coast that has sub-arctic marine environments, south of 75° N on the west coast and of 67° N on the east coast (Rosing-Asvid 2010). Although probably never very numerous, harbour seals were once spread over much of the West Greenland coast. Due to excessive hunting and perhaps interactions with fisheries, harbour seals declined rapidly after the 1950s and disappeared or became extremely rare throughout most of their former range (Rosing-Asvid 2010, Ugarte et al. 2019).
By the early 1990s, most traditional haul-out sites and breeding places had been abandoned. Aerial surveys conducted in 1992 and 1993 suggested that only a handful of former haul-out sites were still in use, and that the numbers of seals at these sites was very low (Teilmann & Dietz, 1994). A rough estimate based on back-calculations, using catch-statistics and typical values of replacement yield, suggest the total number of harbour seals in Greenland to be around 3,000 in 1950 and fewer than 1,000 in 2007 (Rosing-Asvid 2010).
In 2021, breeding and moulting sites of three populations were known. Two of these are located on the west coast of Greenland (around Kangerlussuaq and Majorariaq) and the third is a moulting site on the southeast coast near Cape Farewell, which is used by seals that breed locally and by seals from a breeding site located on the east coast about 250 km north of the moulting site (NAMMCO 2021, Rosing-Asvid et al. 2020).
The number of seals on these known breeding and moulting sites is probably not much more than 100 seals, but observations of individual seals and small groups of seals far from these localities, indicate that other small remnant populations still exist (NAMMCO 2021, Rosing-Asvid 2010, Ugarte et al. 2020). Harbour seals are listed as Critically Endangered in the Greenland Red List in 2018. With the hope of restoring the remnant harbour seal populations, the Greenlandic government imposed a ban on harbour seal hunting in all of Greenland from 1 December 2010 (Anon 2010).
Current research aims to monitor the known sites and to locate the suspected additional sites (Research in NAMMCO Member Countries). The small size and apparent separation of the different groups make them particularly vulnerable to stochastic (chance) events (Blanchet et al. 2021), which remain the primary threat to harbour seals in Greenland.
Iceland
The Icelandic harbour seal population was considerably larger in the early 19th century than at present, with about 60,000 seals (Hauksson and Einarsson 2010a). Aerial surveys conducted since 1980 indicate that the population has declined from 33,000 animals in 1980 to about 11,000 in 2011 (Granquist and Hauksson 2019). The full census of 2016 estimated the population to be about 7,700 animals, which was a decrease of 30% since 2011. The numbers from the 2014 and 2016 censuses suggested that the Icelandic harbour seal population had decreased below the minimum population size of 12,000 seals recommended as a management objective. The result of the full census conducted in 2018 to monitor general and local trends, produced an estimate of 9,434 harbour seals (CI 95% = 6,149–12,726) suggesting that the decline may have halted.
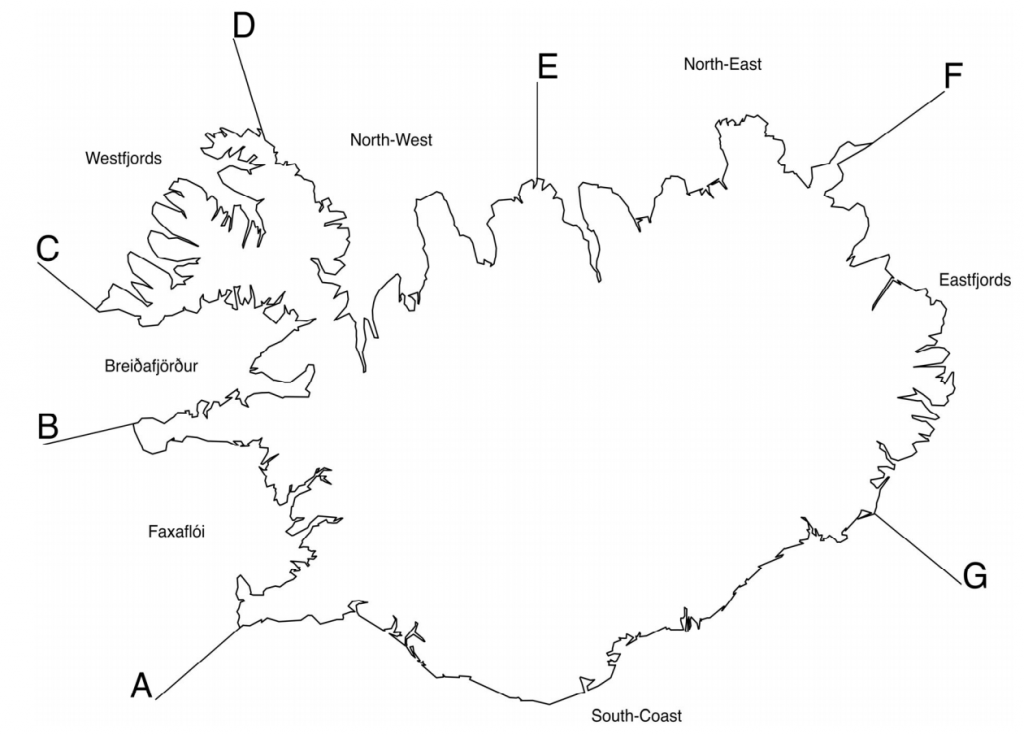
The Icelandic coastline divided into sub‐areas; A – B: Faxaflói, B – C: Breiðafjörður, C – D: Westfjords, D – E: Northwest, E – F: Northeast, F – G: Eastfjords and G – A: South coast (Granquist and Hauksson 2019)
The latest population estimate from 2020 is 10,319 animals (Granquist, 2022), which suggests that the current population is 69.04% smaller than when first estimated in 1980. This corresponds to a decline of 3% annually between 1980 and 2020. The reasons for the decline are poorly understood but may have included a combination of hunting and by-catch, changes in prey availability with important prey species moving northward like sandeels, ecosystem changes related to climate change and warming of the water, diseases and anthropogenic disturbance (Granquist et al. 2019). Another potential factor in the decline of harbour seals is disturbance from tourism. Increasing tourism in Iceland has led to an increase in people visiting remote areas, which can possibly disturb harbour seals during sensitive periods such as the moulting and breeding periods (Granquist and Nilsson 2016). Studies by Burns et al. (2018) and Aquino et al. (2018) examine ethical aspect of the growth in the tourism industry and its impact on wildlife in Iceland.
The annual estimated by-catch of harbour seals in the lumpsucker gillnet fisheries estimated to be over 1,400 seals for the period 2014–2018 (Marine and Freshwater Research Institute 2019). The 23 % population increase between 2016 and 2018 suggests that although by-catch does not hinder the recovery of the populations, it is important to keep monitoring both the population size and by-catch rates, as the populations are small and could be vulnerable to severe by-catch events or changes in the environment (MFRI 2019). Granquist and Hauksson (2019) concluded that because of the sensitive conservation status of the Icelandic harbour seal population, it was urgent to assess the impact of the stressors affecting the population, not only mortality by direct and indirect seal removals, but also climate change, prey availability and disturbance from tourists at haul-out sites, and that actions should be taken to improve the conservation status of the population.
Harbour seals were listed as Critically Endangered in 2018 but as Endangered in 2021 in the Icelandic Red List. Harbour seals became protected in Iceland in 2019. The Marine and Freshwater Institute and the Icelandic Seal Centre continue to closely monitor the harbour seal population.
Faroe Islands

Seal in the harbour in Nólsoy in January 2001 (Photo: Jens -Kjeld Jensen) (Mikkelsen 2010).
Harbour seals were previously common in sheltered fjords in the Faroe Islands, and likely more common than grey seals, but were exterminated in the mid-19th Century through extensive harvesting. Since then, harbour seals have been observed during bounty hunts, in 1889 to 1891 and again in 1963 to 1967 when one and four seals were caught respectively. These animals were caught in the southernmost part of the Southern Island (Bloch et al 2000). Rare observations do still occur, and harbour seals were positively identified in 2001 and 2005 (Mikkelsen 2010) and in 2019 in Tórshavn for a period of a few weeks in August (Faroes National Progress Report 2019).
Should a re-colonisation occur despite the strong natal side fidelity and philopatric nature of harbour seals, it should be monitored, and action taken to protect the animals from hunting and disturbance (NAMMCO 2006).
Norway
Mainland Norway
Harbour seals in Norway suffered a severe decline prior to the 1960s due to unregulated hunting (Øynes 1964). Regulatory measurements were introduced in 1973 in part of the country. After a culling period in 1980–1987, a new management regime with annual quotas was implemented in 1997. Quotas were increased substantially in 2003. A Management Plan was implemented in 2010, with quotas aiming at maintaining the population at a target level of 7000 counted animals during the moult.
Harbour seals are regularly counted along the entire Norwegian coast at known haul-out sites during the moulting period from mid-August to early September. As the Norwegian cost is very long and suitable weather conditions are limited, annual counting surveys cover sub-areas along the coast, which results in a total nation-wide estimate of the number of harbour seal every five years. The most recent survey period (2016–2021) generated a minimum total population of 6,857 harbour seals. Recent surveys have shown an increase in harbour seal abundance in the Skagerrak-Oslofjord area and a decrease in Nordland county, compared to previous counts (NAMMCO 2023).
The population in the outer Oslofjord was severely decimated, and harbour seals on the Skagerrak coast were almost wiped out by the detrimental PDV epizootics in 1988 and 2002. The recent growth is a recovery of the stock and recolonization of former seal rookeries (Nilssen and Bjørge 2019).
Threats to the mainland population are mainly by-catch, with an annual by-catch in the order of 394 (95% CI: 316 – 491) harbour seals (NAMMCO 2021,02023). Until recently, seals could be shot at fish farms, but this was prohibited in November 2019 (executive order FOR-2019-11-28-1593). Some harbour seal areas in Norway may be affected by tourism (e.g., kayaks) (NAMMCO 2016). The Norwegian population is listed as least concern in 2021 in the Norwegian Red List.
Svalbard
The Svalbard harbour seal constitutes the northernmost population of the species, inhabiting a true Arctic environment throughout the year. This distinct population numbers around 1800 individuals (Merkel et al. 2013). The population is protected and there are no gillnet fisheries in the distribution range of this population, which limits the potential for negative human-seal interactions. Concentrations of pollutants in Svalbard harbour seals are much lower than in other populations of this species found further south (Wolkers et al. 2004). However, the low population size, the limited spatial distribution, and the reduced genetic diversity make this population vulnerable to chance events, such as oil spills or disease epidemics (Merkel et al. 2013; Blanchet et al. 2021). The Svalbard population was listed as vulnerable in the Norwegian Red List in 2018 because of its small size, but in 2021 it became listed as near threatened, because of its increase. The species has been protected from hunting since the 1970s.
Management
Harbour seals are under a wide array of conservation status and management regimes across their broad geographic range.
Greenland
There are no quotas for seal hunting in Greenland. Hunting grounds and living resources are open to harvest and use by Greenlandic citizens, subject to hunting licenses (full time or part time license). All catches have to be reported to the Ministry of Fisheries and Hunting.
Until a new online reporting system was implemented in 2013, by-catches of seals and small cetaceans were required to be reported as catches. From April 2016 a new executive order on catch reporting (Government of Greenland Executive Order no. 7 of 4 April 2016 on reporting of purchases of fish and fishery products) made it compulsory for the fishermen and buyers to report all catches (also marine mammals), including by-catch which is not passed on to buyers. It applies to all Greenlandic and foreign vessels operating in Greenlandic waters as well as small vessels below 6m in length. Although harbour seal by-catch happens, by-catch is, not likely of high concern in Greenland (NAMMCO 2017), with few harbour seals reported caught in long lines and in nets for lumpfish and arctic char in the last decade. It may, however, be critical for the survival/recolonisation of small colonies.
In December 2010, noting the decreasing population of harbour seals and on the advice of the Greenland Institute for Natural Resources, the Greenland government imposed a total ban on harbour seal hunting (Anon 2010), with the hope of restoring the remnant harbour seal populations. A few seals may, however, still be caught, as they can be mistaken for young harp seals or taken in nets. Intentional catches have been reported to the police, skins confiscated, and hunters found guilty of illegal hunting (Rosing-Asvid pers. comm.).
The Greenland Institute of Natural Resources continues to closely monitor the harbour seal known haulout sites and to locate the suspected additional sites (Research in NAMMCO Member Countries). The Greenland coastline has been divided into nine tentative management zones on the basis of the available information (typically information from the literature, from interviews of hunters, and field research).
Iceland
Previously, no specific quota system had been established and seal hunting from land and shallow waters was managed by landowners. There was no special protected areas or periods of the year for harbour seals except those imposed by landowners themselves and/or general regulations on hunting. It was not mandatory to report direct seal catches to the government. Members of the Seal Farmers Union (SFU) could voluntarily report their catch statistics to the organization and other known hunters were contacted directly by the Icelandic Seal Centre. The reporting of marine mammal by-catch was and still is mandatory in Iceland.
In 2010, the Icelandic government decided that the management goal for the harbour seal population in Icelandic waters was to keep the population at a minimum number of 12.000 individuals, i.e., the 2006 population level (NAMMCO 2011a [to be found on p. 111], NAMMCO 2011b. The management plan states that management actions should be initiated if the population dropped appreciably below that number, but no specific population regulating method was mentioned and the term “appreciably” was not defined (NAMMCO 2016). Also, the recommended population size was not based on a biological assessment, which NAMMCO recommended it needed to be.
Until 2018, harbour seal culling remained subsidised by the angling industry in an attempt to reduce an assumed predation on salmonids. However, a recent Icelandic study did not find evidence of salmonids in the diet, despite Atlantic salmon (Salmo salar), brown trout (Salmo trutta) and Arctic char (Salvelinus alpinus) being available in the study area (Granquist et al. 2018). Therefore, harbour seals became protected in Iceland in 2019, although they may be hunted under a special licence for traditional use.
Norway
In Svalbard, harbour seals have been protected since the 1970s.
On mainland Norway, the hunt has been regulated by quotas since 1997. In 2003, the quotas and bounties were increased. After a decade with high quotas and a declining population of harbour seals, a management plan was implemented by the Ministry of Fisheries and Coastal Affairs on 5th November 2010, followed by a decrease in the yearly reported catches from 2011. The goal of the Management Plan was to ensure viable populations of harbour seals within their natural distribution areas. A balance between the preservation of large seal populations and reduced damage to fisheries and aquaculture in the coastal zone was desired.
The Management Plan established that the harbour seal population should be stabilised at a level where around 7,000 hauled-out seals can be recorded during the moulting period. The population is maintained at this level through harvest quotas, based on scientific advice provided annually by the Norwegian Marine Mammal Committee after a review of the status in the different management units (last one in 2019 for 2020). Annual counting surveys covering sub-areas along the coast, result in a total nation-wide estimate of the number of harbour seals every five years, which is the basis for recommending quotas. Management units are administrative and follow county borders. Small, unique and geographically isolated populations of harbour seals should not be exposed to hunting. Regulatory management measures for different population sizes in relation to the target level are defined. The administration of hunting licenses and hunting statistics is undertaken by the county authorities (NAMMCO 2016a).
The total annual harvest quota in recent years has been about 450 harbour seals and the reported harvest slightly less, resulting in a population of 7,552 seals over the most recent completed survey period 2011–2015, i.e., slightly higher that the objective of the Management Plan (Bjørge and Nilssen 2019).
Current management units for Norwegian harbour seals are defined by county limits, i.e., are administrative and not biological units. Recent information on the movement patterns of harbour seals in Norway (Bjørge et al. 2002b, Ramasco 2015) and recent genetic evidence of fine scale population structure both in different coastal areas (Olsen et al. 2014, Olsen et al 2017) raise concerns that there may be population subdivisions within counties. Since some counties cover long stretch of coastlines, the present management units are likely larger than the biologically significant populations. There is a risk that small, local, genetic genuine populations can be exterminated (Bjørge and Nilssen 2020). The Institute of Marine Research is therefore studying the genetic population structure along the Norwegian coast with an aim to define biological management units. Preliminary results from genetic samplings indicate local genetic populations in Trøndelag and Nordland counties.
The shooting of seals at fish farms was forbidden in Norway in 2019 (executive order FOR-2019-11-28-1593).
The reporting of by-catch is mandatory for all commercial fishing vessels in Norway, including those from Svalbard. Recreational marine fisheries are important in Norway, with boats numbering in the high hundreds. Recreational boats can carry gillnets with a maximum total length of 210 meters. These gillnets are also likely to catch marine mammals. Therefore, the Directorate of fisheries has recently launched an app for recreational fisheries, “Fritidsfiskeappen”, where reporting of marine mammal by-catch is made easier for recreational fishers through g a dedicated and prominently placed button (Fiskeridirektoratet 2020).
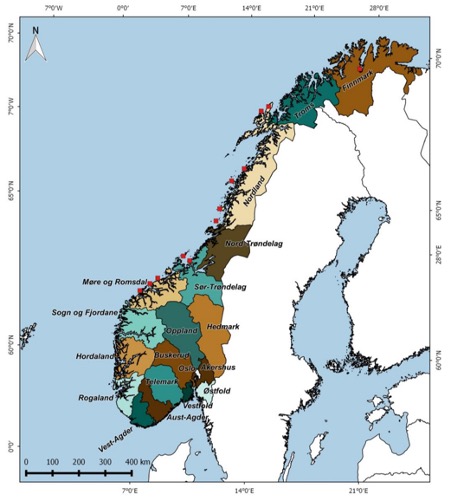
Counties used as management units. Red squares indicate locations where DNA samples were collected (NAMMCO 2016).
Management in bordering areas
Although the harbour seal populations within the remit of NAMMCO management are not shared with outside jurisdictions, a low level of migration may occur.
Canada
The seal harvest is managed as a fishery by the federal government through the department of Fisheries and Oceans (DFO). DFO has published an Integrated Fisheries Management Plan (IFMP) for Atlantic Seals for the period 2011–2015. Its purpose is to identify the main objectives and requirements for the Atlantic seal fishery, as well as the management measures that will be used to achieve these objectives. IFMPs are considered “evergreen” until they are updated or replaced (Lafrance 2017).
Commercial harvesting of harbour seals is not permitted by the Marine Mammal Regulations under the Fisheries Act in the non-Arctic part of Canada (since 1967 for the Pacific population and 1970 for the Atlantic population). In the Arctic, subsistence hunting is permitted for both the marine and freshwater subspecies. The shooting of seals at fish farms was banned in Canada in 2020.
The population of the freshwater subspecies, the Ungava harbour seal, has decreased and counts at the most a few hundred individuals. It was designated as “endangered” by the Committee on the Status of Endangered Wildlife in Canada, COSEWIC, in 2007, and a recovery strategy was defined over 25 years. It is occasionally and opportunistically hunted by the local communities (a few animals a year). The population is monitored regularly, with the impact of the recovery strategy last reviewed in 2018 (DFO 2018).
United Kingdom
In the UK seals are protected under the Conservation of Seals Act 1970 (England, and Wales), the Marine (Scotland) Act 2010 and The Wildlife (Northern Ireland) Order 1985.
Under the Conservation of Seals Act 1970, the UK Natural Environment Research Council (NERC) has a duty to provide scientific advice to the government on matters related to the management of seal populations. NERC has appointed a Special Committee on Seals (SCOS) to formulate this advice. Formal advice is given annually based on the latest scientific information provided to SCOS by the Sea Mammal Research Unit (SCOS 2018).
The harvest of harbour seals is allowed, but is seasonally, regionally and/or quota regulated in England and Wales. Harbour seals may be shot for protection of fishing operations in Scotland (under license), England and Wales. Harbour seals are protected in Northern Irelands (SCOS 2018).
European Union
The ‘Habitats Directive’ (the 1992 Council Directive 92/43/EEC on the Conservation of Natural Habitats and of Wild Fauna and Flora) is the main policy instrument for nature conservation in European waters. Harbour seals are listed as a protected species under Annex II (species requiring the designation of special areas of conservation, SAC, or marine protected areas, MPAs) and V (species whose taking from the wild can be restricted by European law). The monitoring of their population abundance and distribution is requested under the Marine Strategy Framework Directive. In the EU, management regimes involve total protection or controlled hunting regimes for most recognized management units. The species and its habitat are protected and monitored under the 1990 Agreement on the Conservation of Seals in the Wadden Sea The conservation status, abundance, distribution and health of the harbour seal in the Baltic are monitored under the coordination of the Seal Expert Group of the Helsinki Commission (HELCOM), which provides advice on hunting programs, or the restriction of such. Licenses for anthropogenic removals can be issued in most countries.
Hunting and Utilisation
Because of their widespread and coastal presence, harbour seals have been widely and heavily hunted in many areas. Currently they only remain hunted in a few areas, including Norway and Atlantic Canada, but can be culled or shot at fish farms in a few places (See under management).
Greenland
The skin from harbour seals has traditionally been used in the women’s national costume and has therefore been specially coveted (Ugarte et al. 2020). The pups are born with a dense and desirable fur, which has traditionally been used to make trousers in the national costume for women. Pups were therefore the main focus of the hunt, and since 1960, adult seals were protected during May-September (Anon. 1960). The hunt would often take place in or near breeding areas and many of these areas were therefore either depleted by the hunt or abandoned by the seals. However, hunting of this species has been banned in Greenland since 2010. Skins to be used in the national costume have been imported from Iceland, and skins from other species are also now commonly used.
Other harbour seal products were also used for different purposes, human consumption and others, like those of other seal species.
Iceland
Harbour seal is the most numerous seal species in Iceland and has likely been exploited since the settlement of the country. Seal hunting was an important resource for Icelandic farmers for centuries. Between 1962 and 1975, the annual catch was between 4,500 and 6,000 animals, but the market collapsed in 1978 (Hauksson and Einarsson 2010b). Between 1982 and 1989 a bounty system for harbour seals, funded by the Research Committee for Biological Seafood Quality (RCBSQ), was implemented to subsidise sealing and catches increased temporarily. For the last 20 years, harbour seal catches have been relatively small. Recently, harbour seals were mainly hunted around estuaries to decrease suspected seal predation on migrating salmonids, and annual takes ranged from 200–400 harbour seal pups. The hunt in estuary areas was either carried out on behalf of local angling associations or by landowners. The hunting took place in the spring and pups were taken when they were a few weeks old, just towards the end of lactation. Net hunting around the river mouths was the most common method of hunting harbour seal pups. Seal hunting in Iceland did not require a specific hunting license, only a license to own and handle a weapon. Reporting catches was not mandatory (Granquist and Hauksson 2019).
Seal hunting was focused almost entirely on seal pups, mainly for the skin; but the meat, blubber (fat) and flippers have played an important role for human consumption in the past. Harbour seal skins, salted or dried, were typically exported.
The species become protected in 2019 but may be hunted for traditional use under a special licence.
Faroe Islands
Seal hunting, both grey and harbour seals, represented an important food source for the inhabitants of the isolated Faroe Islands. They also provided oil for indoor lighting. Seals were hunted at their haulout and breeding sites and killed with a wooden club. Harbour seals were much easier to hunt because they were re breeding in summer, in easily accessible areas. The number of breeding sites decreased with increasing human activities and the last harbour seals were taken around 1945 (Mikkelsen 2010).
Norway
In Norway, hunting of harbour seals is a regulated recreational hunt. Ideally, seals are shot when they are hauled out. If seals are shot in the water, they need to be retrieved or will otherwise sink and be lost. Hence, this hunt often occurs in shallow waters or with the use of a small vessel as support. The use of rifles with expanding bullets is mandatory.
Harbour seals have been used as food for human consumption and for their skins for thousands of years in Norway. At the Skipshellaren cave in Vaksdal, Western Norway, troglodyte families hunted harbour seals as early as 7200 years ago. Excavations revealed that harbour seal was the second most frequently harvested mammal by these early Norwegians, surpassed only by the red deer. The settlement in Skipshellaren spans a period of about 6000 years.
Petroglyph from South Norway depicting a seal.
Catches in NAMMCO member countries since 1992
| Country | Species (common name) | Species (scientific name) | Year or Season | Area or Stock | Catch Total | Quota (if applicable) |
|---|---|---|---|---|---|---|
| Norway | Harbour seal | Phoca vitulina | 1992-1996 | Norway coast | N/A | |
| Norway | Harbour seal | Phoca vitulina | 2024 | NO / Finnmark | 24 | 60 |
| Norway | Harbour seal | Phoca vitulina | 2024 | NO / Troms | 38 | 40 |
| Norway | Harbour seal | Phoca vitulina | 2024 | NO / Nordland | 65 | 55 |
| Norway | Harbour seal | Phoca vitulina | 2024 | NO / Nord Trøndelag | 0 | No quota |
| Norway | Harbour seal | Phoca vitulina | 2024 | NO / Sør Trøndelag | 20 | 20 |
| Norway | Harbour seal | Phoca vitulina | 2024 | NO / Møre og Romsdal | 19 | 16 |
| Norway | Harbour seal | Phoca vitulina | 2024 | NO / Sogn og Fjordane | 20 | 20 |
| Norway | Harbour seal | Phoca vitulina | 2024 | NO / Hordaland | 0 | No quota |
| Norway | Harbour seal | Phoca vitulina | 2024 | NO / Rogaland | 50 | 50 |
| Norway | Harbour seal | Phoca vitulina | 2024 | NO/Telemark | 16 | 17 |
| Norway | Harbour seal | Phoca vitulina | 2024 | NO /Vestfold | 50 | 50 |
| Norway | Harbour seal | Phoca vitulina | 2024 | NO / Østfold | 52 | 52 |
| Norway | Harbour seal | Phoca vitulina | 2024 | Total | 354 | 380 |
| Iceland | Harbour seal | Phoca vitulina | 2024 | Iceland | 6 | 26 |
| Norway | Harbour seal | Phoca vitulina | 2023 | NO / Finnmark | 22 | 60 |
| Norway | Harbour seal | Phoca vitulina | 2023 | NO / Troms | 36 | 40 |
| Norway | Harbour seal | Phoca vitulina | 2023 | NO / Nordland | 61 | 55 |
| Norway | Harbour seal | Phoca vitulina | 2023 | NO / Nord Trøndelag | 0 | No quota |
| Norway | Harbour seal | Phoca vitulina | 2023 | NO / Sør Trøndelag | 20 | 20 |
| Norway | Harbour seal | Phoca vitulina | 2023 | NO / Møre og Romsdal | 22 | 16 |
| Norway | Harbour seal | Phoca vitulina | 2023 | NO / Sogn og Fjordane | 22 | 22 |
| Norway | Harbour seal | Phoca vitulina | 2023 | NO / Hordaland | 0 | No quota |
| Norway | Harbour seal | Phoca vitulina | 2023 | NO / Rogaland | 14 | 14 |
| Norway | Harbour seal | Phoca vitulina | 2023 | NO/Telemark | 17 | 17 |
| Norway | Harbour seal | Phoca vitulina | 2023 | NO /Vestfold | 50 | 50 |
| Norway | Harbour seal | Phoca vitulina | 2023 | NO / Østfold | 52 | 52 |
| Norway | Harbour seal | Phoca vitulina | 2023 | Total | 316 | 346 |
| Iceland | Harbour seal | Phoca vitulina | 2023 | Iceland | 3 | 41 |
| Greenland | Harbour seal | Phoca vitulina | 2023 | Greenland | 5 | Protected |
| Norway | Harbour seal | Phoca vitulina | 2022 | NO / Finnmark | 23 | 60 |
| Norway | Harbour seal | Phoca vitulina | 2022 | NO / Troms | 24 | 40 |
| Norway | Harbour seal | Phoca vitulina | 2022 | NO / Nordland | 66 | 55 |
| Norway | Harbour seal | Phoca vitulina | 2022 | NO / Nord Trøndelag | 0 | No quota |
| Norway | Harbour seal | Phoca vitulina | 2022 | NO / Sør Trøndelag | 20 | 20 |
| Norway | Harbour seal | Phoca vitulina | 2022 | NO / Møre og Romsdal | 29 | 16 |
| Norway | Harbour seal | Phoca vitulina | 2022 | NO / Sogn og Fjordane | 16 | 22 |
| Norway | Harbour seal | Phoca vitulina | 2022 | NO / Hordaland | 0 | No quota |
| Norway | Harbour seal | Phoca vitulina | 2022 | NO / Rogaland | 16 | 14 |
| Norway | Harbour seal | Phoca vitulina | 2022 | NO /Vestfold & Telemark | 25 | 25 |
| Norway | Harbour seal | Phoca vitulina | 2022 | NO / Østfold | 16 | 16 |
| Norway | Harbour seal | Phoca vitulina | 2022 | Total | 251 | 268 |
| Iceland | Harbour seal | Phoca vitulina | 2022 | Iceland | 6 | 33 |
| Greenland | Harbour seal | Phoca vitulina | 2022 | Greenland | 0 | Protected |
| Norway | Harbour seal | Phoca vitulina | 2021 | NO / Finnmark | 11 | 50 |
| Norway | Harbour seal | Phoca vitulina | 2021 | NO / Troms | 35 | 38 |
| Norway | Harbour seal | Phoca vitulina | 2021 | NO / Nordland | 74 | 55 |
| Norway | Harbour seal | Phoca vitulina | 2021 | NO / Nord Trøndelag | 0 | No quota |
| Norway | Harbour seal | Phoca vitulina | 2021 | NO / Sør Trøndelag | 21 | 20 |
| Norway | Harbour seal | Phoca vitulina | 2021 | NO / Møre og Romsdal | 16 | 16 |
| Norway | Harbour seal | Phoca vitulina | 2021 | NO / Sogn og Fjordane | 23 | 22 |
| Norway | Harbour seal | Phoca vitulina | 2021 | NO / Hordaland | 0 | No quota |
| Norway | Harbour seal | Phoca vitulina | 2021 | NO / Rogaland | 15 | 15 |
| Norway | Harbour seal | Phoca vitulina | 2021 | NO / Vestfold & Telemark | 27 | 25 |
| Norway | Harbour seal | Phoca vitulina | 2021 | NO / Østfold | 16 | 16 |
| Norway | Harbour seal | Phoca vitulina | 2021 | Total | 238 | 257 |
| Iceland | Harbour seal | Phoca vitulina | 2021 | Iceland | 1 | 43 |
| Greenland | Harbour seal | Phoca vitulina | 2021 | Greenland | 0 | Protected |
| Norway | Harbour seal | Phoca vitulina | 2020 | Finnmark | 15 | 75 |
| Norway | Harbour seal | Phoca vitulina | 2020 | Troms | 39 | 75 |
| Norway | Harbour seal | Phoca vitulina | 2020 | Nordland | 202 | 185 |
| Norway | Harbour seal | Phoca vitulina | 2020 | Nord Trondelag | 0 | 0 |
| Norway | Harbour seal | Phoca vitulina | 2020 | Sør Trondelag | 15 | 15 |
| Norway | Harbour seal | Phoca vitulina | 2020 | Møre og Romsdal | 25 | 25 |
| Norway | Harbour seal | Phoca vitulina | 2020 | Sogn og Fjordane | 33 | 32 |
| Norway | Harbour seal | Phoca vitulina | 2020 | Hordaland | 0 | 0 |
| Norway | Harbour seal | Phoca vitulina | 2020 | Rogaland | 17 | 15 |
| Norway | Harbour seal | Phoca vitulina | 2020 | Vestfold & Telemark | 25 | 25 |
| Norway | Harbour seal | Phoca vitulina | 2020 | Østfold | 20 | 20 |
| Norway | Harbour seal | Phoca vitulina | 2020 | Svalbard | ||
| Norway | Harbour seal | Phoca vitulina | 2020 | Total | 391 | 467 |
| Iceland | Harbour seal | Phoca vitulina | 2020 | Iceland | 11 | 67 |
| Greenland | Harbour seal | Phoca vitulina | 2020 | East | 0 | Protected |
| Greenland | Harbour seal | Phoca vitulina | 2020 | West | 1 | Protected |
| Greenland | Harbour seal | Phoca vitulina | 2020 | Total | 1 | |
| Norway | Harbour seal | Phoca vitulina | 2019 | Norway coast | 448 | 476 |
| Iceland | Harbour seal | Phoca vitulina | 2019 | Iceland | 13 | |
| Greenland | Harbour seal | Phoca vitulina | 2019 | East | 0 | Protected |
| Greenland | Harbour seal | Phoca vitulina | 2019 | West | 0 | Protected |
| Greenland | Harbour seal | Phoca vitulina | 2019 | Total | 0 | |
| Norway | Harbour seal | Phoca vitulina | 2018 | Norway coast | 385 | 460 |
| Iceland | Harbour seal | Phoca vitulina | 2018 | Iceland | 43 | |
| Greenland | Harbour seal | Phoca vitulina | 2018 | East | 0 | Protected |
| Greenland | Harbour seal | Phoca vitulina | 2018 | West | 0 | Protected |
| Greenland | Harbour seal | Phoca vitulina | 2018 | Total | 0 | |
| Norway | Harbour seal | Phoca vitulina | 2017 | Norway coast | 338 | 455 |
| Iceland | Harbour seal | Phoca vitulina | 2017 | Iceland | 50 | |
| Greenland | Harbour seal | Phoca vitulina | 2017 | East | 0 | Protected |
| Greenland | Harbour seal | Phoca vitulina | 2017 | West | 0 | Protected |
| Greenland | Harbour seal | Phoca vitulina | 2017 | Total | 0 | |
| Norway | Harbour seal | Phoca vitulina | 2016 | Norway coast | 362 | 455 |
| Iceland | Harbour seal | Phoca vitulina | 2016 | Iceland | 148 | |
| Greenland | Harbour seal | Phoca vitulina | 2016 | East | 1 | Protected |
| Greenland | Harbour seal | Phoca vitulina | 2016 | West | 3 | Protected |
| Greenland | Harbour seal | Phoca vitulina | 2016 | Total | 4 | |
| Norway | Harbour seal | Phoca vitulina | 2015 | Norway coast | 297 | |
| Iceland | Harbour seal | Phoca vitulina | 2015 | Iceland | 159 | |
| Greenland | Harbour seal | Phoca vitulina | 2015 | East | 0 | Protected |
| Greenland | Harbour seal | Phoca vitulina | 2015 | West | 3 | Protected |
| Greenland | Harbour seal | Phoca vitulina | 2015 | Total | 3 | |
| Norway | Harbour seal | Phoca vitulina | 2014 | Norway coast | 409 | |
| Iceland | Harbour seal | Phoca vitulina | 2014 | Iceland | 203 | |
| Greenland | Harbour seal | Phoca vitulina | 2014 | East | 1 | Protected |
| Greenland | Harbour seal | Phoca vitulina | 2014 | West | 19 | Protected |
| Greenland | Harbour seal | Phoca vitulina | 2014 | Total | 20 | |
| Norway | Harbour seal | Phoca vitulina | 2013 | Norway coast | 511 | |
| Iceland | Harbour seal | Phoca vitulina | 2013 | Iceland | 306 | |
| Greenland | Harbour seal | Phoca vitulina | 2013 | East | 0 | Protected |
| Greenland | Harbour seal | Phoca vitulina | 2013 | West | 10 | Protected |
| Greenland | Harbour seal | Phoca vitulina | 2013 | Total | 10 | |
| Norway | Harbour seal | Phoca vitulina | 2012 | Norway coast | 355 | |
| Iceland | Harbour seal | Phoca vitulina | 2012 | Iceland | 105 | |
| Greenland | Harbour seal | Phoca vitulina | 2012 | East | 0 | Protected |
| Greenland | Harbour seal | Phoca vitulina | 2012 | West | 0 | Protected |
| Greenland | Harbour seal | Phoca vitulina | 2012 | Total | 0 | |
| Norway | Harbour seal | Phoca vitulina | 2011 | Norway coast | 230 | |
| Iceland | Harbour seal | Phoca vitulina | 2011 | Iceland | 68 | |
| Greenland | Harbour seal | Phoca vitulina | 2011 | East | 10 | Protected |
| Greenland | Harbour seal | Phoca vitulina | 2011 | West | 69 | Protected |
| Greenland | Harbour seal | Phoca vitulina | 2011 | Total | 79* | |
| Norway | Harbour seal | Phoca vitulina | 2010 | Norway coast | 159 | |
| Iceland | Harbour seal | Phoca vitulina | 2010 | Iceland | 67 | |
| Greenland | Harbour seal | Phoca vitulina | 2010 | Total | 26* | Protected |
| Norway | Harbour seal | Phoca vitulina | 2009 | Norway coast | 585 | |
| Iceland | Harbour seal | Phoca vitulina | 2009 | Iceland | 57 | |
| Greenland | Harbour seal | Phoca vitulina | 2009 | Total | 33 | No quota |
| Norway | Harbour seal | Phoca vitulina | 2008 | Norway coast | 900 | |
| Iceland | Harbour seal | Phoca vitulina | 2008 | Iceland | 34 | |
| Greenland | Harbour seal | Phoca vitulina | 2008 | Total | 81 | No quota |
| Norway | Harbour seal | Phoca vitulina | 2007 | Norway coast | 905 | |
| Iceland | Harbour seal | Phoca vitulina | 2007 | Iceland | 72 | |
| Greenland | Harbour seal | Phoca vitulina | 2007 | Total | 86** | No quota |
| Norway | Harbour seal | Phoca vitulina | 2006 | Norway coast | 538 | |
| Iceland | Harbour seal | Phoca vitulina | 2006 | Iceland | 100 | |
| Greenland | Harbour seal | Phoca vitulina | 2006 | Total | 77** | No quota |
| Norway | Harbour seal | Phoca vitulina | 2005 | Norway coast | 614 | |
| Iceland | Harbour seal | Phoca vitulina | 2005 | Iceland | 121 | |
| Greenland | Harbour seal | Phoca vitulina | 2005 | Total | ** | No quota |
| Norway | Harbour seal | Phoca vitulina | 2004 | Norway coast | 549 | |
| Iceland | Harbour seal | Phoca vitulina | 2004 | Iceland | 146 | |
| Greenland | Harbour seal | Phoca vitulina | 2004 | Total | ** | No quota |
| Norway | Harbour seal | Phoca vitulina | 2003 | Norway coast | 467 | |
| Iceland | Harbour seal | Phoca vitulina | 2003 | Iceland | 416 | |
| Greenland | Harbour seal | Phoca vitulina | 2003 | Total | ** | No quota |
| Norway | Harbour seal | Phoca vitulina | 2002 | Norway coast | 412 | |
| Iceland | Harbour seal | Phoca vitulina | 2002 | Iceland | 371 | |
| Greenland | Harbour seal | Phoca vitulina | 2002 | Total | ** | No quota |
| Norway | Harbour seal | Phoca vitulina | 2001 | Norway coast | 466 | |
| Iceland | Harbour seal | Phoca vitulina | 2001 | Iceland | 611 | |
| Greenland | Harbour seal | Phoca vitulina | 2001 | Total | 73 | No quota |
| Norway | Harbour seal | Phoca vitulina | 2000 | Norway coast | 359 | |
| Iceland | Harbour seal | Phoca vitulina | 2000 | Iceland | 656 | |
| Greenland | Harbour seal | Phoca vitulina | 2000 | Total | 124 | No quota |
| Norway | Harbour seal | Phoca vitulina | 1999 | Norway coast | 308 | |
| Iceland | Harbour seal | Phoca vitulina | 1999 | Iceland | 649 | |
| Greenland | Harbour seal | Phoca vitulina | 1999 | Total | 148 | No quota |
| Norway | Harbour seal | Phoca vitulina | 1998 | Norway coast | 83 | |
| Iceland | Harbour seal | Phoca vitulina | 1998 | Iceland | 566 | |
| Greenland | Harbour seal | Phoca vitulina | 1998 | Total | 217 | No quota |
| Norway | Harbour seal | Phoca vitulina | 1997 | Norway coast | 60 | |
| Iceland | Harbour seal | Phoca vitulina | 1997 | Iceland | 694 | |
| Greenland | Harbour seal | Phoca vitulina | 1997 | Total | 295 | No quota |
| Iceland | Harbour seal | Phoca vitulina | 1996 | Iceland | 850 | |
| Greenland | Harbour seal | Phoca vitulina | 1996 | Total | 255 | No quota |
| Iceland | Harbour seal | Phoca vitulina | 1995 | Iceland | 865 | |
| Greenland | Harbour seal | Phoca vitulina | 1995 | Total | 266 | No quota |
| Iceland | Harbour seal | Phoca vitulina | 1994 | Iceland | 1039 | |
| Greenland | Harbour seal | Phoca vitulina | 1994 | Total | 278 | No quota |
| Iceland | Harbour seal | Phoca vitulina | 1993 | Iceland | 1196 | |
| Iceland | Harbour seal | Phoca vitulina | 1992 | Iceland | 1149 |
This database of reported catches is searchable and the information can be filtered, e.g. by country, species or area. It is also possible to sort it by the different columns, in ascending or descending order. The number of entries shown can also be changed in the drop-down menu.
Carry-over from previous years are included in the quota numbers, where applicable.
The full catch database with all species is available here.
Other Human Impacts
Harbour seals occupy coastal habitats in close proximity to human populations, they are therefore exposed in many areas to a variety of humans’ activities besides harvest, such as interactions with fisheries, population reduction programs, coastal development, agricultural runoff and pollution, seal watching and other recreational activities. The effects of each of these stressors is not well understood, especially as their cumulative impact might not be simply additive but synergistic (see more under cumulative impacts).
By-catch and entanglements
Harbour seals frequent good fishing grounds to forage and are particularly exposed to entanglement in gillnets. This makes by-catch likely the major threat to harbour seal populations in the North Atlantic. Typically, large-meshed nets, such as those used for monkfish (Lophius piscatorius), lump sucker (Cyclopterus lumpus) and cod (Gadus morhua) fisheries, tend to have the greatest seal by-catch rates.
Estimating the exact level of by-catch is challenging for several reasons. There are two main ways to obtain by-catch data: self-reporting by fishermen through logbooks and observer programs. The reporting of by-catch of marine mammals is mandatory for most commercial fisheries in NAMMCO member countries. However, as in other countries, marine mammal by-catch incidents are not usually reported, even in fisheries that have known by-catch. The NAMMCO Scientific Committee Working Group on By-catch, concurring with other expert groups, reiterates the unreliability of using self-reporting for by-catch estimation (NAMMCO 2017, 2018ab, 2020) On the other hand though, observer programs are costly to implement for relatively rare events, as high effort is required to obtain reliable estimates.
Recreational fisheries, especially gillnet fisheries, are also potentially by-catching smaller marine mammals, however reporting is not mandatory and not common. Recreational marine fisheries are important in most NAMMCO member countries. In Norway, boats are in high hundreds and can carry gillnets (with a maximum total length of 210 meters), the most problematic gear in relation to by-catch. Therefore, the Directorate of fisheries has recently launched an app for recreational fisheries, “Fritidsfiskeappen”, where reporting of marine mammal by-catch is made easy by which featuring a dedicated and prominently placed button (Fiskeridirektoratet 2020).
In the specific case of seals, there is also a strong potential for misidentification of species, particularly for young seals. Young grey and harp seals (Pagophilus groenlandicus) can easily be mistaken for harbour seals and reported as such. Some countries are presently trying to minimise this problem by adding photos and identification guides to fishery logbooks as well as through analysis of DNA samples of the by-caught seals.
In Greenland, a few harbour seals have been reported by-caught in long lines and in nets for lumpfish and arctic char in the last decade. By-catch is not likely of high concern for the overall population but might be critical for the survival/recolonisation of small colonies (NAMMCO 2017, Rosing-Asvid pers. comm.).
In the Faroe Island waters, by-catch should be very limited as there is a maximum of 4 Faroese gillnetters operating (1 vessel fishing for monkfish and 2–3 for Greenland halibut) and no foreign gillnet fisheries (NAMMCO 2020).
In Iceland, reliable by-catch data comes from the observer programs (1% coverage of the fleet and representative geographical spread) and the annual March–April research survey. The fisheries of most concern regarding harbour seal by-catch are primarily the lumpsucker gillnet fishery and to a, likely, lesser degree, the cod gillnet fisheries, although information is limited for some fisheries (NAMMCO 2016, 2017, 2018ab, 2020). Around 1400 harbour seals are estimated to be by-caught annually in lumpsucker gillnets in the period 2014–2018. To these numbers should be added the by-catch in the cod gillnet fishery, which remains difficult to assess due to the lack of data outside of March–April (MFRI 2019). Due to the high estimate of harbour seals drowning in fishing gear around Iceland, attempts have been made recently to reduce local seal by-catch (Granquist, 2022). Experimental closures have been enacted in marine areas of the lumpsucker fishery where bycatch risks are highest. Further, preliminary experiments have also been made with pingers on fishing gear (audio deterrent devices, ADDs) with the purpose of reducing bycatch (NAMMCO, 2020).
In Norway, reliable marine mammal by-catch data are obtained from a coastal reference fleet (CRF), which has been operating since 2006 in the cod and monkfish gillnet fisheries, the two fisheries where the by-catch appeared to be the most severe (Moan & Bjørge 2020b). By-catch is significantly higher in the monk-fish fishery, with the highest by-catch rates in July–December. Although analysis remains ongoing and improvements in precision are still being made, preliminary estimates suggest a by-catch in Norwegian gillnet fisheries of around 394 harbour seals each year (NAMMCO 2023).
Conflicts with fisheries & aquaculture
Interactions with fish farming and angling
In Iceland, interactions between seals and fish farms are reduced with using double-netting. There are still some interactions between harbour seals and salmon fisheries around the river mouths, but removals are thought to be low, about 2–3 per year. The culling of seals at estuaries for reducing salmonids predation became prohibited in 2019.
In Norway, killing seals near aquaculture facilities became prohibited in 2019 (Executive order FOR-2019-11-28-1593).
Competition with fisheries
Bounty systems were historically in place in Canada, the United States, Iceland, Norway and Sweden to control local harbour seal populations in areas where fisheries and angling took place. This was to reduce competition with the fisheries. As the Skagerrak and Kattegat population of harbour seals has recently increased, claims have emerged that seals are depleting coastal cod populations. The estimated population size in the Norwegian Skagerrak shows an increase from 350 animals in 1996–1999 to 880 in 2016–2018 (Nilssen and Bjørge 2019). The diet of harbour seals in Norwegian Skagerrak was investigated based on otolith identification from scats (Sorlie et al. 2020). Fish length estimates showed that seals generally prefer small fish below the minimum allowed landing size. The estimated total amount of fish consumed was 315 tons per year and was dominated by non-commercial species. Annual cod consumption was an estimated 7.1 tons, representing 5% of the commercial fishery annual cod landings, to which should be added the non negligeable landing by recreational fisheries. The study therefore suggested that harbour seals do not constitute a serious direct competition with local fisheries along the Norwegian Skagerrak coast, and that predation by seals does not constitute an important factor in preventing the recovery of the depleted coastal cod stocks.
Vectors of parasite infestation in fish
Bounty systems in Iceland and Norway also aimed to control the incidence of roundworm in commercial fish. Harbour seals are infested by larval stages of a parasitic nematode from the food they eat. This nematode matures in the intestinal tract of the seals. Eggs are transported into the sea water with the seal’s faeces. The eggs sink to the sea floor and hatch. Larval stages occur in small benthic crustaceans and in fish feeding on these crustaceans. In the fish, the larval parasites penetrate the intestinal wall and migrate into the muscles (fillets) of the fish where they incapsulate and wait for the fish to be eaten by a seal so the life cycle can be completed. These nematodes in the fillets have a detrimental effect on the value of commercial fish on the fresh fish market. This is an important conflict with the coastal fisheries (Bjørge and Nilssen 2020).
Tourism, marine traffic and noise
Commercial shipping, vessel-based tourism, seal watching and increased urbanisation are sources of disturbance for harbour seal colonies, causing potential shifts in local distribution and population decline (Reijnders 1994, Jansen et al. 2015, Granquist and Nilsson 2016). Under water noise may create a long-term noise-induced permanent threshold shift in a harbour seal, i.e., short but also long-term hearing loss (Reichmuth et al. 2019).
In several areas harbour seals have been found stranded with peculiar injuries consisting of a single continuous curvilinear skin laceration spiralling down the body. After some investigation, the cause of death was found to be a traumatic event, with the seal being drawn through the ducted propellers of marine vessels (Bexton et al. 2012).
Pollutants
Pollutants are well known to negatively impact marine mammals. Previously the levels of pollutants in harbour seals were generally regarded as low and not constituting a health threat (Drescher et al. 1977). However, some environmental contaminants (especially persistent organic pollutants and mercury) are known to affect the immune system and might play a role in rendering marine mammal populations vulnerable to disease through immunotoxicity (Mos et al. 2006).
Southern harbour seal populations in proximity to human settlements typically carry heavier pollutant loads compared to their northern counterparts. Animals from Svalbard contain 5–10 times less contaminants compared to seals in the Norwegian mainland and their concentrations are 30 times lower than those of harbour seals from more industrialized areas such as in the Gulf of St Lawrence (Wolkers et al. 2004). A more recent study confirms a precipitous decline in legacy persistent organic pollutants (POPs), with a drop in concentrations of 60–90 % over the period of a decade (Routti et al. 2014). Both these studies conclude that the current levels of the studied contaminants are not a threat to harbour seals on Svalbard, but that potential signs of endocrine disruption should be closely monitored.
Climate change
Warming is occurring more rapidly in the Arctic than in any other region on the globe and predictions suggest that the degree of change is expected to be the greatest in this ecosystem (Kovacs and Lydersen 2008, Onerheim et al. 2014).
Northwards range shifts of prey species are not only affecting predator-prey relationships but also pathogen-host dynamics (Tryland et al. 2009, Kovacs et al. 2012). Pathogens such as viruses, bacteria and parasites can be transmitted to new immunologically naive host populations via invasive species, seasonal migrations, migrations caused by nutritional stress or changes in the host population behaviour (Tryland et al. 2009). Pathogens tend to generally grow faster and survive better when temperatures are warmer and may exhibit a higher survival rate in a warming environment that was previously too cold. Changes in haulout patterns due to the reduction of availability of sea ice platforms may also increase the risk of direct contact between different species, favouring the transmission of pathogens.
Harbour seals have not been a typical prey for polar bears in Svalbard, although it has been common in Greenland. In recent years predation by polar bears on harbour seals has been documented in Svalbard (Lydersen pers. comm.). The predation pressure of polar bears may generally increase as they spend more time on land and ringed seals, an ice-dependent species, may become less abundant because of ice reduction.
In the perspective of global climate change, harbour seals are particularly unique as ecosystem sentinels due to their broad distribution in the northern hemisphere and the variety of environmental conditions this species encounters. This offers the possibility for worldwide inter-population comparison and for identifying drivers influencing population dynamics (Blanchet et al. 2021). The case of the northernmost harbour seal population on Svalbard is especially interesting because this species is essentially temperate, not ice-affiliated, and is not a true Arctic species. It is possible that they might have a competitive advantage over local arctic species under warmer conditions (Blanchet 2015).
Greenland
Site monitoring and exploration
Harbour seals are rare in Greenland. There are probably less than 200 seals associated with the three known breeding/moulting localities. Research currently aims to monitor the known sites and to locate the suspected additional sites, since observations of harbour seals far from known sites indicate that there may be some additional small breeding and moulting sites not yet detected. An ongoing interview study to assist with this was initiated in West Greenland in 2019, to map all former, still inhabited and potentially new sites (National Progress Report Greenland 2019, 2020).
Seal movements, dive patterns and behaviour
The first telemetry study of deep diving harbour seals in South Greenland deployed 15 satellite-linked data-recorders on 12 individual harbour seals at moulting sites in the southern tip of Greenland near Cape Farewell across 2 years (Rosing-Asvid et al. 2020). This study thoroughly described their movements, dive patterns, haul-out behaviour, and breeding activities. The data collected in this study provides a new dive depth record, with a male reaching 631m. The research also presents a novel way to estimate pregnancy status, position and date of parturition based on telemetry data. The study found that an abrupt reduction of home range and simultaneous changes in both the dive and the haulout patterns reflected parturition. The results also suggest that the harbour seals in Southern Greenland could consist of two distant and separated breeding populations that share the same foraging and moulting area outside the breeding season. The satellite tagging of harbour seals continues and has generated interesting questions about the functional significance and specificity of moulting and breeding sites, especially when they can be long distances apart.
Iceland
In recent years, research in Iceland has focused on getting updated abundance estimates, information on diet and prey selection, genetic and contaminant analysis, as well as information on movements and foraging behaviour in estuaries. A master’s project on vocalisations and behaviour of male Icelandic harbour seals during the mating season continued through 2020 (National Progress Report Iceland 2019, 2020).
Population Decline
The factors, and potentially the combination of them, that caused the decline in the population continued to being investigated further by researchers in Iceland at the Marine and Freshwater Institute (MFRI) and the Icelandic Seal Centre (ISC). Additionally, work continues towards obtaining reliable estimates of seal by-catch.
Aerial surveys & haul-out behaviour
Census of seals are regularly conducted in Iceland. A project monitoring haul-out behaviour using camera traps was initiated in 2018. This ongoing project will assist in improving census design by increasing the knowledge of factors affecting haulout behaviour. A new census was conducted during the moulting period of 2020 in a collaboration between MFRI and ISC. Estimated population sizes for respective year from 1980 to 2020 are available in Granquist (2022).
Effect of seal tourism
In addition, a study on the effect of land- and boat-based tourism on the spatial and behavioural haul-out patterns of harbour seals was initiated by ISC and MFRI in 2018 and continued during 2020. This study includes cooperation with researchers from Holar University Collage, University of Iceland, Griffith University in Australia, Stockholm University in Sweden. This interdisciplinary research focuses on best practices, management of seal watching and development of an ethical framework (National Progress Report Iceland 2020).
Norway
Surveys
Regular counts for abundance are carried out along the entire coast of Norway. Visual counts using binoculars from small boats and land are carried out in areas not covered by aerial surveys, or where results from the aerial surveys seem to be incomplete. In 2016, electric helicopter drones with cameras were added to these survey efforts.
Satellite tagging
Between 2017 and 2022, a total of 28 harbour seals were tagged with GPS-phone tags in the Oslofjord-Skagerrak area, with tags providing data for up to 178 days, and a total of 98 864 GPS positions. Results indicated that seals moved both within and between all five counties in Norwegian Skagerrak: Østfold, Vestfold, Telemark, Aust-Agder and Vest-Agder. In addition, movement connectivity was found between colonies in Norway, Sweden and Denmark, indicating the need to revise current harbour seal management units (NAMMCO, 2023).
Population Structure
The harbour seal population is managed county by county. These administrative management units are not based on science. There is a risk that small, local, genetic genuine populations can be wiped out. The Institute of Marine Research is therefore studying the genetic population structure along the Norwegian coast with the aim of defining biological management units. Preliminary results from genetic sampling indicates local genetic populations in Trøndelag and Nordland counties.
DNA samples from harbour seal pups were taken in 2020 and sampling will be continued in 202, to explore potential genetic segregations along the coast north of Stad (62°N) (National Progress Report Norway 2020).
References
Andersen, L.W., Lydersen, C., Frie, A.K., Rosing-Asvid, A., Hauksson, E. and Kovacs, K.M. (2011). A population on the edge: genetic diversity and population structure of the world’s northernmost harbour seals (Phoca vitulina). Biological Journal of the Linnean Society, 102, 420–439. https://doi.org/10.1111/j.1095-8312.2010.01577.x
Andersen, L. and Olsen, M.T. (2010). Distribution and population structure of North Atlantic harbour seals (Phoca vitulina). NAMMCO Scientific Publications, 8, 15–35. https://doi.org/10.7557/3.2669
Anon. (1960). Landsrådsvedtægt af 31.august 1959, stadfæstet den 12 februar 1960, om fredning af spraglet sæl. – Grønlandsk Lovsamling, Serie A nr. 1:19-20. [Law regarding regulation of the harbour seal hunt]. (In Danish).
Anon. (2010). “Selvstyrets bekendtgørelse nr. 16 af 12. november 2010 om beskyttelse og fangst af sæler” [The Greenland Government executive order no. 16 dated 12. November 2010 about protection and hunting of seals].
Aquino, J.F., Burns, G.L. and Granquist, S.M. (2018). Seal Watching in Iceland: Ethical Management Development. In J. Dehex (ed.), The 9th International Conference on Monitoring and Management of Visitors in Recreational and Protected Areas (MMV9): Place, Recreation, and Local Development (pp. 165–167). Bordeaux, France. Retrieved from https://mmv9.sciencesconf.org/data/pages/last_version_abstract_book_7.pdf
Bexton, S., Thompson, D., Brownlow, A., Barley J, Milne, R. and Bidewell, C. (2012). Unusual Mortality of Pinnipeds in the United Kingdom Associated with Helical (Corkscrew) Injuries of Anthropogenic Origin. Aquatic Mammals, 38, 229–240. https://doi.org/10.1578/AM.38.3.2012.229
Bjørge, A., Bekkby, T., Bryant, E.B. (2002). Summer home range and habitat selection of harbour seal (Phoca vitulina) pups. Marine Mammal Science, 18, 438–454.
Bjørge, A., Thompson, D., Hammond, P., Fedak, M., Bryant, E., Aarefjord, H., Roen, R., Olsen, M. (1995). Habitat use and diving behaviour of harbour seals in a coastal archipelago in Norway. In Blix, A.S., Walløe, L. and Ulltang, Ø. (eds.), Whales, seals, fish and man, 211–223. Tromsø: Elsevier Science.
Bjørge, A., Desportes, G., Waring, G.T. and Rosing-Asvid, A. (2010). Introduction: The harbour seal (Phoca vitulina) – a global perspective. NAMMCO Scientific Publications, 8, 7-11. https://doi.org/10.7557/3.2668
Bjørge, A. and Nilssen, K.T. 2020. Topic: Harbour seals. Institute of Marine Research. https://www.hi.no/en/hi/temasider/species/harbour-seals
Blanchet M.-A., Lydersen C., Ims, R. and Kovacs K.M. (2015). Seasonal oceanographic and atmospheric drivers of diving behaviour in a temperate seal species living in the High Arctic. PLOS ONE. doi: 10.1371/journal.pone.0132686
Blanchet, M.-A.; Lydersen, C.; Ims, R. A.; Lowther, A. D.; Kovacs, K. M. (2014). Harbour Seal Phoca Vitulina Movement Patterns in the High-Arctic Archipelago of Svalbard, Norway. Aquatic Biology, 21(3). https://doi.org/10.3354/ab00580.
Blanchet, M.-A., Vincent, C., Womble, J.N., Steingass, S.M. and Desportes, G. (2021). Harbour Seals: Population Structure, Status, and Threats in a Rapidly Changing Environment. Oceans, 2.
Bloch, D., Mikkelsen, B. and Ofstad, L.H. (2000). Marine Mammals in Faroese Waters – with Special Attention to the South-South-Eastern Sector of the Region. Available at: http://projects.foib.fo/eia/Faroe_eia/Studies/Mammal_Final_Part1.pdf.
Bowen, W.D. and Harrison, G.D. (1996). Comparison of harbour seal diets in two inshore habitats of Atlantic Canada. Canadian Journal of Zoology, 74, 125–135. https://doi.org/10.1139/z96-017
Bowen, W.D., Tully, D., Boness, D.J., Bulheier, B.M. and Marshall, G.J. (2002). Prey-dependent foraging tactics and prey profitability in a marine mammal. Marine Ecology Progress Series, 244, 235–245. https://doi.org/10.3354/meps244235
Bowen, W.D., McMillan, J. and Mohn, R. (2003). Sustained exponential population growth of grey seals at Sable Island, Nova Scotia. ICES Journal of Marine Science, 60, 1265–1274. https://doi.org/10.1016/S1054-3139(03)00147-4
Boyd, I.L. (1991). Environmental and physiological factors controlling the reproductive cycles of pinnipeds. Canadian Journal of Zoology, 69, 1135–1148. https://doi.org/10.1139/z91-162
Boulva, J., and. McClaren, I.A. (1979). Biology of the harbor seal, Phoca vitulina, in eastern Canada. Bulletin of Fisheries Research Board of Canada, 200, 24 p.
Burns, G.L., Öqvist, E.L., Angerbjörn, A. and Granquist, S. (2018). When the wildlife you watch becomes the food you eat: Exploring moral and ethical dilemmas when consumptive and non-consumptive tourism merge. In Kline, C. (ed), Animals, food, and tourism (pp. 22-35). New York: Routledge Ethics of tourism. https://doi.org/10.4324/9781315265209-3
Colominas, R. (2012). Harbour seal diet in a changing Arctic (Svalbard, Norway). MSci. thesis, University of Bergen, Norway, 47 pp.
Crocker, D.E., LeBoeuf, B.J. and Costa, D.P. (1997). Drift diving in female northern elephant seals: implications for food processing. Canadian Journal of Zoology, 75, 27–39. https://doi.org/10.1139/z97-004
Cronin, M. (2010). A modelling framework to optimize timing of haulout counts for estimating harbour seal (Phoca vitulina) abundance. NAMMCO Scientific Publications, 8, 213–226. https://doi.org/10.7557/3.2686
Cronin, M., Zuur, A., Rogan, E. and McConnell, B. (2009). Using mobile phone telemetry to investigate the haul-out behaviour of harbour seals Phoca vitulina vitulina. Endangered Species Research, 10, 255–267. https://doi.org/10.3354/esr00170
DFO. (2018). Recovery Strategy for the Harbour Seal, Lacs des Loups Marins subspecies (Phoca vitulina mellonae). Species at Risk Act Recovery Strategy Series. Fisheries and Oceans Canada, Ottawa. v +28 pp.
Dietz, R., Teilmann, J., Andersen, S.M., Rige, F. and Olsen, M.T. (2013). Movements and Site Fidelity of Harbour Seals (Phoca Vitulina) in Kattegat, Denmark, with Implications for the Epidemiology of the Phocine Distemper Virus. ICES Journal of Marine Science, 70, 186–195.
Dietz R, Teilmann J, Andersen SM, Rigét F, Olsen MT (2012) Movements and site fidelity of harbour seals (Phoca vitulina) in Kattegat, Denmark, with implications for the epidemiology of the phocine distemper virus. ICES Journal of Marine Science, 69, 1–10.
Drescher, H.E., Harms, U. and Huschenbeth, E. (1977). Organochlorines and heavy metals in the harbour seal Phoca vitulina from the German North Sea Coast. Marine Biology, 41, 99–106. https://doi.org/10.1007/BF00390586
Duignan, P. J., Bressem, M.-F.V., Baker, J.D., Barbieri, M., Colegrove, K.M., Guise, S.D., de Swart, R.L. et al. (2014). Phocine Distemper Virus: Current knowledge and future directions. Viruses, 6(12), 5093–5134.
Enns, A., Kraus, D. and Hebb, A. (2020). Ours to save: the distribution, status and conservation needs of Canada’s endemic species. NatureServe Canada and Nature Conservancy of Canada. https://www.natureconservancy.ca/assets/documents/nat/Ours-to-Save_NCC_NatureServe_Jun4_2020.pdf
Fiskeridirektoratet. (2020). Ny app for fritidsfiske. Retrieved from https://www.fiskeridir.no/Fritidsfiske/Nyheter/2020/Ny-app-for-fritidsfiske
Gilbert, J.R., Waring, G.T., Wynne, K.M. and Guldager, N. (2005). Changes in abundance of harbor seals in Maine, 1981–2001. Marine Mammal Science, 21, 519–535. https://doi.org/10.1111/j.1748-7692.2005.tb01246.x
Granquist, S.M. and Hauksson, E. (2016). Seasonal, meteorological, tidal and diurnal effects on haul-out patterns of harbour seals (Phoca vitulina) in Iceland. Polar Biology, 39, 2347–2359. https://doi.org/10.1007/s00300-016-1904-3
Granquist, S.M., Esparza-Salas, R., Hauksson, E., Karlsson, O. and Angerbjörn, A. (2018). Fish consumption of harbour seals (Phoca vitulina) in north western Iceland assessed by DNA metabarcoding and morphological analysis. Polar Biology, 41, 2199–2210. https://doi.org/10.1007/s00300-018-2354-x
Granquist, S.M. and Hauksson, E. (2019). Population estimate, trends and current status of the Icelandic harbour seal (Phoca vitulina) population in 2018. Marine and Freshwater Research Institution, HV 2019-36. Reykjavík. 22pp. https://www.hafogvatn.is/static/research/files/1575899073-hv2019-36.pdf
Granquist, S. M. (2022). The Icelandic harbour seal (Phoca vitulina) population: trends over 40 years (1980–2020) and current threats to the population. NAMMCO Scientific Publications 12. https://doi.org/10.7557/ 3.6328
Granquist, S.M., and Nilsson, P.Å. (2016). Who’s watching whom? – an interdisciplinary approach to the study of seal-watching tourism in Iceland. Journal of Cleaner Production, 111(B), 471–478 https://doi.org/10.1016/j.jclepro.2014.11.060
Hall, A.J., Watkins, J. and Hammond, P.S. (1998). Seasonal variation in the diet of harbour seals in the south-western North Sea. Marine Ecology Progress Series, 170, 269–281. https://doi.org/10.3354/meps170269
Hammill, M.O., Bowen, W.D. and Sjare, B. (2010). Status of harbour seals (Phoca vitulina) in Atlantic Canada. NAMMCO Scientific Publications, 8, 175–189. https://doi.org/10.7557/3.2684
Hamilton, C.D., Lydersen, C., Ims, R.A. and Kovacs, K.M. (2014). Haul-Out Behaviour of the World’ s Northernmost Population of Harbour Seals (Phoca Vitulina) throughout the Year. PLoS, 9(1), e86055. https://doi.org/10.1371/journal.pone.0086055.
Hauksson, E. and Einarsson, S.T. (2010a). Historical trend in harbour seal (Phoca vitulina) abundance in Iceland back to the year 1912. NAMMCO Scientific Publications, 8, 147–159. https://doi.org/10.7557/3.2682
Hauksson, E. and Einarsson, S.T. (2010b). Review on utilization and research of harbour seal (Phoca vitulina) in Iceland. NAMMCO Scientific Publications, 8, 341–354. https://doi.org/10.7557/3.2698
Härkönen, T. (1987). Seasonal and regional variations in the feeding -habits of the harbor seal, Phoca vitulina, in the Skagerrak and the Kattegat. Journal of Zoology, 213, 535–543. https://doi.org/10.1111/j.1469-7998.1987.tb03724.x
Härkönen, L., Dietz, R., Reijnders, P.J.H., Teilmann, J., Harding, K., Hall, A., Brasseur, S.M.J.M., Siebert, U., Goodman, S.J., Jepson, P.D., Rasmussen, T.D. and Thompson, P. (2006). A review of the 1988 and 2002 phocine distemper virus epidemics in European harbour seals. Diseases of Aquatic Organisms, 68, 115–130. https://doi.org/10.3354/dao068115
Härkönen, T. and Isakson, E. (2010). Status of harbour seals (Phoca vitulina) in the Baltic proper. NAMMCO Scientific Publications, 8, 71–76. https://doi.org/10.7557/3.2673
Härkönen, T. and K. C. Harding (2010). Predicting recurrent PDV epizootics in European harbour seals (Phoca vitulina). NAMMCO Scientific Publications, 8, 275–284. https://doi.org/10.7557/3.2690
ICES. (2017). Report of the workshop on predator‐prey interactions between grey seals and other marine mammals (WKPIGS), 30 April 2017. Middelfart, Denmark, ICES Cm 2017/SSGEPD:18.
Jansen, J.K., Boveng, P.L., Ver Hoef, J.M., Dahle, S.P. and Bengtson, J.L. (2015). Natural and human effects on harbor seal abundance and spatial distribution in an Alaskan glacial fjord. Marine Mammal Science, 31, 66–89. https://doi.org/10.1111/mms.12140
Johnston, D.W., Frungillo, J., Smith, A., Moore, K., Sharp, B., Schuh, J. and Read, A.J. (2015). Trends in stranding and by-catch rates of gray and harbor seals along the Northeastern coast of the United States: Evidence of divergence in the abundance of two sympatric phocid species? PLoS ONE, 10(7), e0131660. https://doi.org/10.1371/journal.pone.0131660
Jones, E.L., McConnell, B.J., Smout, S., Hammond, P.S., Duck, C.D., Morris, C.D., Thompson, D., Russell, D.J.F., Vincent, C., Cronin, M., Sharples, R.J. and Matthiopoulos, J. (2015). Patterns of space use in sympatric marine colonial predators reveal scales of spatial partitioning. Marine Ecology Progress Series, 534, 235–249. https://doi.org/10.3354/meps11370
Jones, E.L., Sparling, C.E., McConnell, B. J., Morris, C.D., Smout, S. (2017). Fine-Scale Harbour Seal Usage for Informed Marine Spatial Planning. Scientific Reports, 7(1), 11581. https://doi.org/10.1038/s41598-017-11174-4
Leclerc, L.-M. E., C. Lydersen, T. Haug, L. Bachman, A. T. Fisk and K. M. Kovacs (2012). A missing piece in the Arctic food web puzzle? Stomach contents of Greenland sharks sampled in Svalbard, Norway. Polar Biology, 35, 1197–1208. https://doi.org/10.1007/s00300-012-1166-7
Lesage, V., Hammill, M.O. and Kovacs, K.M. (2004). Long-distance movements of harbour seals (Phoca vitulina) from a seasonally ice-covered area, the St. Lawrence River estuary, Canada. Canadian Journal of Zoology, 82, 1070–1081. https://doi.org/10.1139/z04-084
Kovacs, K.M. and Lydersen, C. (2008). Climate change impacts on seals and whales in the North Atlantic Arctic and adjacent shelf seas. Science Progress, 91, 117–150. https://doi.org/10.3184/003685008X324010
Lafrance, D. (2017). Canada’s seal harvest (Background Paper). [Canadian] Library of Parliament. Publication no. 2017-18-E. 11pp.
Lydersen, C. and Kovacs, K.M. (2005). Growth and population parameters of the world’s northernmost harbour seals Phoca vitulina residing in Svalbard, Norway. Polar Biology, 28, 156–163. https://doi.org/10.1007/s00300-004-0656-7
Lydersen, C. and Kovacs, K.M. (2010). Status and biology of harbour seals (Phoca vitulina) in Svalbard. NAMMCO Scientific Publications, 8, 47–60. https://doi.org/10.7557/3.2671
Marine and Freshwater Research Institute (MFRI). (2019). Bycatch of seabirds and marine mammals in lumpsucker gillnets 2014-2018. 18pp. [In Icelandic at https://www.hafogvatn.is/static/extras/images/medafli-fugla-og-spendyra-i-grasleppuveidum1158397.pdf ]
Merkel, B., Lydersen, C., Yoccoz, N.G. and Kovacs, K.M. (2013). The World’s Northernmost Harbour Seal Population – How Many Are There ? PLoS One, 8(7). https://doi.org/10.1371/journal.pone.0067576
Middlemas, S.J., Barton, T.R., Armstrong, J.D. and Thompson, P.M. (2006). Functional and aggregative responses of harbour seals to changes in salmonid abundance. Proceedings of the Royal Society B Biology Science, 273, 193–198. https://doi.org/10.1098/rspb.2005.3215
Mikkelsen, B. (2010). A note on the harbour seal (Phoca vitulina) in the Faroe Islands. NAMMCO Scientific Publications, 8, 143–146. https://doi.org/10.7557/3.2681
Mizuno, M., Kobayashi, M., Sasaki, T., Haneda, T. and Masubuchi, T. Current population genetics of Japanese harbor seals: Two distinct populations found within a small area. Marine Mammal Sciences, 36, 915–924. https://doi.org/10.1111/mms.12697
Mos, L., Morsey, B., Jeffries, S.J., Yunker, M.B., Raverty, S., De Guise, S. and Ross, P.S. (2006). Chemical and biological pollution contribute to the immunological profiles of free-ranging harbor seals. Environmental Toxicology and Chemistry, 25(12), 3110–3117. https://doi.org/10.1897/06-027R.1
Moan, A. and Bjørge, A. (2020a) Bycatch estimates of harbour (Phoca vitulina) and grey (Halichoerus grypus) seal in Norwegian gillnet fisheries. NAMMCO SC/27/BYC/06 for the NAMMCO Scientific Committee Working Group on By-Catch.
Moan, A., Skern-Mauritzen, M., Vølstad, J. H., and Bjørge, A. (2020b). Assessing the impact of fisheries-related mortality of harbour porpoise (Phocoena phocoena) caused by incidental bycatch in the dynamic Norwegian gillnet fisheries. ICES Journal of Marine Science, fsaa186. https://doi.org/10.1093/icesjms/fsaa186
Muelbert, M.M.C. and Bowen, W.D. (1993). Duration of lactation and postweaning changes in mass and body composition of harbour seal, Phoca vitulina, pups. Canadian Journal of Zoology, 71, 1405–1414. https://doi.org/10.1139/z93-194
Nilssen, K.T. and Bjørge, A. 2019. Status for kystsel: Anbefaling av jaktkvoter for 2019 [Status of coastal seals: recommendation of hunting allowances for 2020]. Norway. Sjøpattedyrutvalget [Committee for Marine Mammals], Institute of Marine Research.
https://www.fiskeridir.no/Yrkesfiske/Dokumenter/Hoeringer/Jakt-paa-kystsel-i-2020
North Atlantic Marine Mammal Commission (NAMMCO). (2020). Report of the By-Catch Working Group. Available at: https://nammco.no/topics/byc_reports/
North Atlantic Marine Mammal Commission (NAMMCO). (2018ab). Report of the NAMMCO Scientific Committee on By-Catch. Available at: https://nammco.no/topics/byc_reports/
North Atlantic Marine Mammal Commission (NAMMCO). (2017). Report of the NAMMCO Scientific Committee on By-Catch. Available at: https://nammco.no/topics/byc_reports/
North Atlantic Marine Mammal Commission (NAMMCO). (2016). Report of the NAMMCO Scientific Committee on Coastal Seals. Available at: https://nammco.no/topics/cswg_reports/
North Atlantic Marine Mammal Commission (NAMMCO). (2011a) Report of the Management Committee for Seals and Walruses. Page 105-121. In NAMMCO (ed.) Annual Report 2010. 501pp.
North Atlantic Marine Mammal Commission (NAMMCO). (2011b). Report of the NAMMCO Scientific Committee on Coastal Seals. Available at: https://nammco.no/topics/cswg_reports/
North Atlantic Marine Mammal Commission (NAMMCO). (2006). Report of the NAMMCO Scientific Committee Working Group on Harbour Seals. Available at: https://nammco.no/topics/cswg_reports/
NAMMCO-North Atlantic Marine Mammal Commission (2021). Report of the Scientific Committee Working Group on By-Catch. Available at: https://nammco.no/topics/byc_reports/
NAMMCO-North Atlantic Marine Mammal Commission (2023). Report of the Scientific Committee Working Group on Coastal Seals. Available at: https://nammco.no/topics/cswg_reports/
North Atlantic Marine Mammal Commission (NAMMCO). (2015). Report of the Committee on Hunting Methods. Available at: https://nammco.no/topics/committee-on-hunting-methods/
Olsen, M.T., Andersen, S.M., Teilmann, J., Dietz, R., Edrén, S.M.C., Linnet, A. and Härkönen, T. (2010). Status of the harbour seal (Phoca vitulina) in Southern Scandinavia. NAMMCO Scientific Publications, 8, 77–94. https://doi.org/10.7557/3.2674
Olsen, M.T., Andersen, L.W., Dietz, R., Teilmann, J., Härkönen, T. and Siegismund, H.R. (2014). Integrating genetic data and population viability analyses for the identification of harbour seal (Phoca vitulina) populations and management units. Molecular Ecology, 23, 815–831.
Olsen, M.T., Islas, V., Graves, J.A., Onoufriou, A., Vincent, C., Brasseur, S., Frie, A.K. and Hall, A.J. (2017). Genetic population structure of harbour seals in the United Kingdom and neighbouring waters. Aquatic Conservation: Marine and Freshwater Ecosystems, 27(4), 839–845. https://doi.org/10.1002/aqc.2760
Olsen, M. and Bjørge, A. (1995). Seasonal and regional variations in the diet of harbour seal in Norwegian waters. In Blix AS, Walloe L, Ulltang Ø (eds.), Whales, seals, fish and man, 271–285. Amsterdam: Elsevier. https://doi.org/10.1016/S0163-6995(06)80030-2
Onerheim, I. H., Smedsrud, L.H., Ingvaldsen R.B. and Nilsen, F. (2014). Loss of sea ice during winter north of Svalbard. Tellus, 66, 23933.
Pierce, G.J., Thompson, P.M., Miller, A., Diack, J.S.W., Miller, D. and Boyle, P.R. (1991). Seasonal-variation in the diet of common seals (Phoca-vitulina) in the Moray-Firth area of Scotland. Journal of Zoology, 223, 641–652. https://doi.org/10.1111/j.1469-7998.1991.tb04393.x
Ramasco, V. (2015). Spatial and temporal patterns of foraging of harbour seals (Phoca vitulina) in Porsangerfjord, from behavioural interpretation to resource selection. PHD thesis. UiT, the Arctic University of Norway. https://munin.uit.no/handle/10037/8149
Ramasco, V., Biuw, M. and Nilssen, K.T. (2014). Improving time budget estimates through the behavioural interpretation of dive bouts in harbour seals. Animal Behaviour, 94, 117–134. https://doi.org/10.1016/j.anbehav.2014.05.015
Ramasco, V., Barraquand, F., Nilssen, K.T. and Mcconnell, B. (2013). Identifying periods of “resting” at sea helps making sense of harbour seals’ foraging signature in movement data. In Proceeding of the 20th Biennial Conference on the Biology of Marine Mammals. Dunedin (NZ)
Ramasco, V., Lindström, U. and Nilssen, K.T. (2017). Selection and foraging response of harbour seals in an area of changing prey resources. Marine Ecology Progress Series, 581, 199–214. https://doi.org/10.3354/meps12285
Reichmuth, C., Sills, J.M., Mulsow, J. and Ghoul, A. (2019). Long-term evidence of noise-induced permanent threshold shift in a harbor seal (Phoca vitulina). The Journal of the Acoustical Society of America, 146, 2552, https://doi.org/10.1121/1.5129379
Reijnders, P.J.H. (1994). Historical population size of the harbour seal, Phoca vitulina, in the Delta area, SW Netherlands. In P.H. Nienhuis and A.C. Smaal (eds.), The Oosterschelde Estuary (The Netherlands): a Case-Study of a Changing Ecosystem, 557–560. Dordrecht: Springer . https://doi.org/10.1007/978-94-011-1174-4_40
Reijnders, P.J.H., Brasseur, S.M.J.M. and Meesters, E.H.W.G. (2010). Earlier pupping in harbour seals, Phoca vitulina. Biology Letters, 6(6). https://doi.org/10.1098/rsbl.2010.0468
Reijnders, P.J.H., Brasseur, S.M.J.M., Tougaard, S., Seibert, U., Borchardt, T. and Stede, M. (2010). Population development and status of harbour seals (Phoca vitulina) in the Wadden Sea. NAMMCO Scientific Publications, 8, 95–105. https://doi.org/10.7557/3.2677
Rohner S, Hülskötter K, Gross S, Wohlsein P, Abdulmawjood A, Plötz M, Verspohl J, Haas L, Siebert U. (2020). Male grey seal commits fatal sexual interaction with adult female harbour seals in the German Wadden Sea. Scientific Reports, 10(1), 13679. https://doi.org/10.1038/s41598-020-69986-w
Rosing-Asvid, A. (2010). Catch history and status of the harbour seal (Phoca vitulina) in Greenland. NAMMCO Scientific Publications, 8, 161-174. https://doi.org/10.7557/3.2683
Rosing-Asvid, A., Teilmann, J., Dietz, R. and Olsen, M. (2010). First Confirmed Record of Grey Seals in Greenland. Arctic, 63(4). https://doi.org/10.14430/arctic3336
Rosing-Asvid, A., Teilmann, J., Olsen, M.T. and Dietz, R. (2020). Deep diving harbor seals (Phoca vitulina) in South Greenland: movements, diving, haul‑out and breeding activities described by telemetry. Polar Biology, 43, 359–368. https://doi.org/10.1007/s00300-020-02639-w
Routti, H., Lydersen, C., Hanssen, L. and Kovacs, K.M. (2014). Contaminant levels in the world’s northernmost harbor seals (Phoca vitulina). Marine Pollution Bulletin, 87, 140–146.
Sjare, B., Lebeuf, M. and Veinott, G. (2005). Harbour seals in Newfoundland and Labrador: a preliminary summary of new data on aspects of biology, ecology and contaminant profiles. DFO Canadian Science Advisory Secretariat. Res. Doc. 2005/030. http://www.dfo-mpo.gc.ca/csas-sccs/publications/resdocs-docrech/2005/2005_030-eng.htm
Special Committee on Seals (SCOS). (2018). Scientific advice on matters related to the management of seal populations: 2018. Available online: http://www.smru.st-andrews.ac.uk/files/2019/05/SCOS-2018.pdf
Sharples, R.J., Moss, S.E., Patterson, T.A. and Hammond, P.S. (2012). Spatial variation in foraging behaviour of a marine top predator (Phoca vitulina) determined by a large-scale satellite tagging program. PLoS One, 7, e37216. https://doi.org/10.1371/journal.pone.0037216
Sørlie, M., Nilssen, K.T., Bjørge, A. and Freitas, C. (2020). Diet composition and biomass consumption of harbour seals in Telemark and Aust-Agder, Norwegian Skagerrak. Marine Biology Research, 16(4), 299-310. https://10.1080/17451000.2020.1751205
Teilmann, J. and Dietz, R. (1994). Status of the harbor seal, Phoca vitulina, in Greenland. Canadian Field-Naturalist 108, 139–155.
Teilmann, J. and Galatius, A. (2018). Harbor Seal. In B. Würsig, J. G. M. Thewissen, and K.M. Kovacs (eds.), Encyclopedia of Marine Mammals, 451–455. (Third, Edition). Academic Press.
Thomas, A., Lance, M., Jeffries, S., Miner, B. and Acevedo-Gutiérrez, A. (2011). Harbor seal foraging response to a seasonal resource pulse, spawning Pacific herring. Marine Ecology Progress Series, 441, 225–239. https://doi.org/10.3354/meps09370
Thompson, D., Duck, C., Lonergan, M.E. (2010). The status of harbour seals (Phoca vitulina) in the United Kingdom. NAMMCO Scientific Publications, 8, 117–127. https://doi.org/10.7557/3.2679
Tollit, D.J., Black, A.D., Thompson, P.M., Mackay, A., Corpe, H.M., Wilson, B., Van Parijs, S.M., Grellier, K. and Parlane, S. (1998). Variations in harbour seal Phoca vitulina diet and dive-depths in relation to foraging habitat. Journal of Zoology, 244, 209–222. https://doi.org/10.1111/j.1469-7998.1998.tb00026.x
Tougaard, J., Teilmann, J. and Tougaard, S. (2008). Harbour seal spatial distribution estimated from Argos satellite telemetry: Overcoming positioning errors. Endangered Species Research, 4, 113–122. https://doi.org/10.3354/esr00068
Ugarte, F., Rosing-Asvid, A., Heide-Jørgensen, M.P. and Laidre, K.L. (2020). Marine Mammals of the Greenland Seas. In M.I. Goldstein and D.A. DellaSala (eds.), Encyclopedia of the World’s Biomes, 575-586. https://doi.org/10.1016/B978-0-12-409548-9.12485-6
Van Neer, A., Jensen, L.F. and Siebert U. (2015). Grey seal (Halichoerus grypus) predation on harbour seals (Phoca vitulina) on the island of Helgoland, Germany. Journal of Sea Research, 97, 1–4. https://doi.org/10.1016/j.seares.2014.11.006
Van Parijs, S.M., Thompson, P.M., Tollit, D.J. and Mackay, A. (1997). Distribution and activity of male harbour seals during the mating season. Animal Behaviour, 54, 35–43. https://doi.org/10.1006/anbe.1996.0426
Vincent, C., McConnell, B. J., Delayat, S., Elder, J.-F., Gautier, G. and Ridoux, V. (2010). Winter Habitat use of harbour seals (Phoca Vitulina) fitted with Fastloc(™) GPS/GSM tags in two tidal bays in France. NAMMCO Scientific Publications, 8, 285–302. https://doi.org/10.7557/3.2691
Waring, G.T., DiGiovanni Jr, R.A., Josephson, E., Wood, S. and Gilbert. J.R. (2015a). 2012 Population estimate for the harbor seal (Phoca vitulina concolor) in New England waters. US Department Of Commerce, NOAA Technical Memorandum NMFS-NE-235, 15p.
Waring, G.T., Josephson, E., Maze-Foley, K. and Rosel, P.E. (eds.). (2015b). US Atlantic and Gulf of Mexico Marine Mammal Stock Assessments — 2014. NOAA Tech Memo NMFS NE 231; 361 p. https://repository.library.noaa.gov/view/noaa/5043
Waring, G.T., Gilbert, J.R., Belden, D., Van Atten, A. and DiGiovanni Jr, R.A. (2010). A review of the status of harbour seals (Phoca vitulina) in the Northeast United States of America. NAMMCO Scientific Publications, 8, 191–212. https://doi.org/10.7557/3.2685
Wilson, L., & Hammond, P. (2016). Harbour seal diet composition and diversity. Marine mammal scientific Support Research Programme MMSS/001/11 CSD 3.2. Report to the Scottish Government. Scottish Marine and Freshwater Science Report, 7(21). https://data.marine.gov.scot/sites/default/files/SMFS%20Vol%207%20No%2021.pdf
Wilson, L.J. and Hammond, P.S. (2019). The diet of harbour and grey seals around Britain: Examining the role of prey as a potential cause of harbour seal declines. Aquatic Conservation: Marine Freshwater Ecosystem, 29(S1), 71–85. https://doi.org/10.1002/aqc.3131
Wolkers, H., C. Lydersen and K. M. Kovacs (2004). Accumulation and lactational transfer of PCBs and pesticides in harbor seals (Phoca vitulina) from Svalbard, Norway. Science of the Total Environment, 319, 137–146. https://doi.org/10.1016/S0048-9697(03)00449-2
Øynes, P. (1964). Sel på norskekysten fra Finnmark til Møre. Fiskets Gang, 50, 694–707.
Þorbjörnsson, J.G., Hauksson, H., Sigurðsson, G.M. and Granquist, S.M. (2017). Aerial census of the Icelandic harbour seal (Phoca vitulina) population in 2016: Population estimate, trends and current status. Marine and Freshwater Research Institute, Reykjavik, Iceland. Available at https://www.researchgate.net/publication/315110781