Long-finned Pilot Whale
Long-finned Pilot Whale
Latest update: July 2021
The long-finned pilot whale is a medium-sized toothed whale that is found in temperate and sub polar areas of the North Atlantic and in mid-latitudes throughout the Southern Hemispheres. It is primarily an oceanic, squid-eating species. Males are larger than females, reaching a length of 6.3 m and a weight of 2.5 tonnes. They are dark brown to black in colour, with a light anchor-shaped pattern on the belly. The pilot whale is a social species, found in groups of tens to hundreds of animals.

[The information presented on this page focuses on stocks within the remit of NAMMCO, including stocks shared with non-NAMMCO member countries]
ABUNDANCE
A combination of surveys in 2015 produced a minimum abundance estimate of 380,000 whales for an area covering the northernmost pilot whale distribution area in the North east Atlantic.
From surveys in 1989, 1995, and 2007, there were over 100,000 in the eastern index area, which includes the Faroe Islands.
DISTRIBUTION
In the subpolar and temperate waters of the NAMMCO area, ranging in the summer months from Disco Bay in western Greenland, to Iceland and up to the southern portion of Svalbard waters and down to northwest Africa.
Most common in the Irminger Sea and in the area between Iceland and Scotland and Ireland during summer.
RELATION TO HUMANS
Average annual catches since 2000:
- Faroe Islands = 632 (from 0 to 1,203 up to and including 2020),
- Greenland = 248 (from 5 to 433, up to and including 2018), mainly in West Greenland.
CONSERVATION and MANAGEMENT
The Faroese drive fishery is considered sustainable, although the last comprehensive assessment of the stock dates back to 1997 (NAMMCO 1998b). In 2012, a partial assessment concluded that sustaining an annual catch of 678 animals (the average annual catch for the 1997–2011 period) required a target population of 50,000–80,000 pilot whales. The population sustaining the catch is estimated to be over 100,000 whales (NAMMCO 2013).
A full assessment of the sustainability of both the Faroese and Greenlandic catches is planned for 2025. It will integrate the latest survey results from 2015 and the upcoming survey in 2024, and the trend analysis, as well as the latest tagging and genetic information.
The species is listed as ‘Least Concern’ on the regional European IUCN Red List (2023) and on the Norwegian and Icelandic Red Lists. It is protected in Norway and Iceland.

A fast swimming long-finned pilot whale © A. Li, NOAA/NMFS/SWFSC
Names
Scientific name: Globicephala melas (Trail 1809)
Faroese: Grindahvalur
Greenlandic: Niisarnaq
Icelandic: Marsvin, grindahvalur
Norwegian: Grindhval
Danish: Grindehval
English: Long-finned pilot whale, blackfish, pothead, caaing whale, pothead whale
Pilot whales get their name from the original belief by the inhabitants of the Orkney, Shetland and Faroe Islands that there was a “pilot” or lead individual within a group. They used that knowledge to drive them into shallow bays to hunt them (Lesson 1828). The name for the genus, “Globicephala” is derived from a combination of the Latin words globus (“globe”) and kephale (“head”). The specific name “melas” is Greek for “black”.
Average size
552 cm in length and 1.7 tons for adult males and 432 cm and 0.9 tons for adult females.
Lifespan
Average of 59 years in females and 46 years in males.
Productivity
One calf every 5 years from 9 years of age on average (range 6–15 years).
Feeding
Primarily schooling squid in mid water, with some preferred species when available, but also small pelagic fish.
Migration
No definite migration patterns, but movements associated with prey sources, as in the Faroes, where the abundance of pilot whale seems to follow that of the European flying squid.
General Characteristics
The long-finned pilot whale is a medium sized toothed whale, belonging to the family of oceanic dolphins, also called the delphinid family.
Long-finned pilot whales are dark brown to black in colour, with younger animals being paler and new-borns being light grey. There is a light anchor-shaped pattern on the belly, and on some animals, a whitish stripe extends towards the tail along the back and sometimes also behind the dorsal fin. The flippers are long (up to one-fifth of the body length or more), sickle-shaped and acutely pointed at the tip. The tail is dorsally thickened just in front of the flukes, which have a concave trailing edge and a deep median notch.
At sea
The long-finned pilot whale is a very social species and is almost always seen in groups of tens or hundreds of animals of both sexes, but single animals are also observed. They have a thick and bulbous head, but the dorsal fin of the adults is their most distinctive feature. It is falcate (hooked) to flaglike, low in profile, markedly longer at the base than at the peak and set far forward on the animal’s back. This makes pilot whales easy to differentiate from any other species in the NAMMCO area. The two species they could be confused with, the short-finned pilot whale and the false killer whale (Pseudorca crassidens), overlap at the southern end of the range but are not common in the NAMMCO area.
The blow of the pilot whale is usually inconspicuous. Pilot whales only rarely show surface behaviours when swimming. Occasionally they can be seen resting or ‘logging’ at the surface, and sometimes spy hopping or lobtailing. Their dark colour and lack of aerial behaviour make them relatively inconspicuous at sea, unless they occur in a large group. They are relatively slow-swimming; radio-tagged individuals averaged a speed of 3.3 km/hr, with burst of up to 16 km/hr (Mate 1989), while the average speed of three satellite-tagged whales off the Faroes was 4.7 km/hr (Bloch et al. 2003).
Pilot whales are commonly observed in association with other species such as common bottlenose (Tursiops truncatus), common (Delphinus delphis) and Atlantic white-sided (Lagenorhynchus acutus) dolphins, but can also be observed with fin (Balaenoptera physalus), minke (Balaenoptera acutorostrata) and sperm whales (Physeter macrocephalus) and Risso’s dolphins (Grampus griseus).
Size
Off the Faroe Islands, males reach a maximum length of 6.25 m and a weight of 2.3 tonnes, compared to 5.12 m and 1.3 tonnes for females. Calves average 178 cm (range: 163–191 cm) at birth (Bloch et al. 1993b). Pilot whales exhibit striking sexual dimorphism. In addition to their larger size, adult males develop a more pronounced and bulbous melon and have a much larger and rounder dorsal fin with a thicker leading edge. They also have longer flippers and larger flukes (Bloch et al. 1993c).
Subspecies and congeneric species
Three subspecies are recognised (Rice 1998, Oremus et al. 2009, Committee on Taxonomy 2017):
- m. edwardii (Smith, 1834) living in the southern oceans,
- m. melas (Traill, 1809) in the North Atlantic and Mediterranean Sea.
- An un-named subspecies in Japanese waters (extinct since the 8th–12th century A.D).
The gene flow (i.e., contact) between the northern and southern subspecies is highly restricted (Oremus et al. 2009) and they show significant differences in skull morphology (Marina et al. 2019). The Southern Hemisphere sub-species is more oceanic and occurs far offshore but can also be found in coastal areas.
Long-finned and short-finned pilot (G. macrorhynchus) whales differ in their flipper length, skull shape and number of teeth (Bloch et al. 1993c, Culik 2011) and are therefore difficult to distinguish at sea. The range of long-finned pilot whales overlaps spatially with the range of its congeneric (belonging to the same genus) species, the short-finned pilot whale, at its southern edge, but short-finned pilot whales are not common in the NAMMCO area (Olson 2018). Hybridisation of the two species appears to be uncommon but has been documented (Miralles et al. 2013).
Behaviour
Social behaviour
Like many species of toothed whales, pilot whales are highly social animals and usually occur in pods of 10 to 200 whales, with larger pods occurring more rarely. The largest pod caught in the Faroese drive fishery numbered 1200 animals (Zachariassen 1993, Bloch 1998). Large pods are also observed in other areas (Cañadas and Sagarminaga 2000, de Stephanis et al. 2008). Pilot whales show a hierarchical social structure with a population composed of a number of clans, each containing several pods. Pods are formed by several matrilineal units (Verborgh 2015). Pods usually comprise animals of all ages and sexes, with adult females outnumbering adult males (Bloch et al. 1993a). However male-only pods and pods consisting mainly of males have been observed (Desportes et al. 1992a), indicating that some sex segregation can occur. All individuals in the pod, including adult males, are related, although. However, males tend not to be the fathers of the calves in the pod, and the pods constitute close matrilineal associations (Amos et al. 1991, 1993ab, Anderson 1990, 1993). Investigation in other areas, based on photo-identification and genetic work, confirms the existence of relatively stable pods, like those of killer whales (Orcinus orca) and unlike the fluid groups characteristic of many smaller dolphins (Jefferson et al. 1993, Canadas and Sagarminaga 2000, Ottensmeyer and Whitehead 2003, Stephanis et al. 2008, Verborgh 2015).
The highly synchronous breathing and diving observed between close pairs of individuals of a specific matrilineal unit likely help to maintain and reinforce the strong social bonds observed (Senigaglia et al. 2012, Aoki et al. 2013), as does the prolonged lactation period (Desportes and Mouritsen 1993).

A group of pilot whales, including a large male and a calf seen during NASS 95. Note the much paler colour of the calf (bottom), the bulbous head of the large male (top) and its dorsal fin very low in profile and with a particularly thick leading edge. © Geneviève Desportes
Pilot whales are loosely polygynous (i.e., males mate with multiple females but females only mate with a single male). Mating occurs reciprocally between pods and there is no evidence of strong male reproductive dominance (Amos et al. 1993a). Males do not necessarily leave their pods, but may mate with females from other pods during aggregation of different clans (Amos et al. 1993b, Andersen and Siegismund 1994, Cañadas and Sagarminaga 2000). Individual males may sire several offspring in the same pod (Amos et al. 1991).
Surface and diving behaviour
Long-finned pilot whales can exhibit highly synchronous breathing and diving behaviour, both during shallow and deep dives, at less than one body length apart and coupled with body contact (Senigaglia and Whitehead 2012, Aoki et al. 2013). Synchronous swimming does not save locomotion effort, but rather on the contrary, entails some locomotion costs and must therefore serve other functions, such as reinforcing social bonds (Senigaglia et al. 2012, Aoki et al. 2013). Close proximity also allows for a rapid coordinated response to disturbance (Senigaglia et al. 2012). This social and synchronised foraging strategy can be maintained during deep foraging dives, when pilot whales jointly swim to several hundred meters depth in search of prey (Aoki et al. 2013), although groups may spread out during foraging and individuals may not be synchronised for all their dives (Visser 2014, Visser et al 2014).
In Norway, echolocation clicks to locate prey were recorded on all dives below 34 m but prey capture, indicated by a high number of buzzes, occurred between 400 and 848 m (Visser et al. 2014). In the Mediterranean Sea, pilot whales take advantage of the prey, such as squid, moving closer to the surface at night (following the raising of the deep scattering layer), and indeed forage for more hours during longer winter nights (Baird et al. 2002, Giorli et al. 2016).
An original diving and foraging behaviour?
Diving behaviour was studied off the Faroe Islands by equipping pilot whales with satellite-linked time-depth recorders (Heide-Jørgensen et al. 2002, Bloch et al. 2003, Mikkelsen 2008 and pers. comm.) and off Norway by using Dtags (sound and movement recording tags) (Sivle et al. 2012). Dives may last up to 18 min to a maximum depth of 852 m, although 60% of the dives lasted less than 3 minutes. Most diving activity occurred at depths of less than 36 m, even if the whales were also travelling off the shelf over water depths exceeding 1,500 m. The whales spent 60% of their time above 7 m. In both areas, pilot whales exhibited this intermediate diving behaviour, with the animals spending most of their time close to the surface but typically conducting bouts of foraging dives to intermediate depth of 300–600 m. In the Mediterranean dives reached 648 m for a maximum of 12.7 min (Baird et al. 2002), while in the northwest Atlantic, dives lasted longer, up to 28 min, but were shallower with a maximum depth of 320 m (Nawojchik et al. 2003, Mate et al. 2005).
Compared to other odontocetes of similar size, the long-finned pilot whale spent a higher proportion of their time at the surface and exhibited less extreme diving in terms of duration and depth. This may be linked to lower dive capability and/or the use of a foraging niche in the water column requiring less extreme diving. They may also have a foraging behaviour similar to short-finned pilot whales and use deep sprints for catching prey capable of moving fast, such as Todarodes (flying squid). Sprints challenge the common view on optimal foraging in breath-holding, deep-diving predators, as it represents a high-cost foraging strategy. However it may be necessary to secure the capture of high-value, but evasive and rapid prey (Aguilar de Soto et al. 2008).
Acoustic behaviour

A fast swimming long-finned pilot whale © A. Li, NOAA/NMFS/SWFSC
The acoustic behaviour of pilot whales has been quite extensively studied, and in particular off Norway. Pilot whales produce clicks, buzzes, whistles, pulse sounds and calls that can vary depending on their behaviour. They have one of the more complex vocal repertoires among mammalian species (Taruski 1979, Vester et al. 2017). Vocalisations serve to identify individuals, maintain contact, and coordinate and synchronise the movements of the herd (Taruski 1979, Weilgart and Whitehead 1990, Kok 2012, Zwamborn and Whitehead 2017). The vocalisations are more complex and more numerous while the animals are foraging and with increasing dispersal of the pod or more subgroups present. During resting or travelling, pilot whales are less vocal. They can be almost silent while resting at the surface, which points to the importance of vocalisation in the synchronisation of behaviour. There seem to be a vocal adaptation to background noise, with calls in noisier conditions having a significantly higher pitch, longer duration and higher inflection rates (Rendell et al. 1999).
The clicks of pilot whales have a mean apparent source level of 196 dB re 1 µPa pp, a duration of 23 μs, (S.E. = 1.3) and a centroid frequency at 55 kHz (S.E. = 2.1); they are different in duration (shorter), frequency (higher), and energy distribution (lower) than those of killer whales (Orcinus orca) in the same area (Eskesen et al. 2011).
The calls of the long-finned and short-finned pilot whales are distinct despite their close relatedness, and similar size and morphology, which may be due to selection pressures to maintain distinctiveness (Steiner 1981, Rendell et al. 1999). This feature could help distinguish the two species during surveys in areas where they overlap, since it is almost impossible to distinguish them visually at sea.
Multi-species interactions
Pilot whales are one the most abundant cetacean species in the North Atlantic, and their impact on the ecosystem as consumers is likely substantial. In the central North Atlantic around Iceland, pilot whales are the third most important marine mammal predator in terms of total biomass, consuming close to 300,000 tonnes of fish, and 1100 thousand tonnes of squid per year (Sigurjónsson and Víkingsson 1997). They are also one of the most important marine mammal predators in the western North Atlantic (Gannon et al. 1997a,b).
Feeding at a lower trophic level than most other toothed whales or spatially segregating from other cephalopod–eating species may alleviate inter-specific competition (Verborgh and Desportes 2021).
Predation
Besides humans, pilot whales have few predators besides killer whales and large sharks. Striking changes in behaviour have been observed during playback of killer whale sounds to pilot whales (Miller et al. 2011, Curé et al. 2012, Visser et al. 2016), which might be able to discriminate between the sounds of fish and mammal eating killer whales (Visser et al. 2016, Curé et al. 2019).
Parasites and diseases
In the northeastern Atlantic and the Mediterranean Sea, long-finned pilot whales can be infested by at least 18 species of endo- and ectoparasites (Verborgh and Desportes 2021). Pilot whales are often infested with whale lice, cestodes, trematodes, nematodes and acanthocephala (Balbuena and Raga 1991, 1993, Raga and Balbuena 1993). Whale lice have been found in larger numbers on males, associated with wounds that could have resulted from intrasexual competition (Raga and Balbuena 1993). The stomachs of Faroese-caught pilot whales are frequently and massively infested with the nematode Anisakis simplex, the infestation beginning at the onset of feeding and increasing with age (Raga and Balbuena 1993). Some of these parasites can cause pathological lesions such as ulcers on the stomach wall (A. simplex), or infestation of the mammary glands (Crassicauda sp.), potentially lowering milk production and therefore reproductive success (Balbuena and Raga 1993).
Respiratory bacterial pathogens have been described in free ranging pilot whales (Verborgh and Desportes 2021).
Mass Strandings
Pilot whales have a propensity to mass-strand throughout their range, including in the Central and Northeast Atlantic where groups of mainly healthy animals have stranded on sandy beaches (Bloch et al. 1993a, Olson 2018). It is unknown whether human activity influences these events, and at present, such strandings must be considered a component of natural mortality. Different hypotheses have been developed (Olson 2018) but the strong social bonds between individuals of a pod and the leading behaviour are believed to be important factors. Human and military activity producing loud underwater noise, such as the use of high frequency sonar, could be involved on some occasions (Culik 2011, Brownlow et al. 2015).
In the NAMMCO area, mass strandings of pilot whales are recurrent in Iceland, with four events in the last 40 years, two of them occurring in the summer of 2019 (Sigurjónsson et al. 1993, Víkingsson pers. comm.). In total, 280 pilot whales have died in a mass-stranding since 1982, the largest involving at least 148 animals in 1986. Typically, only a minority of the animals (if any) can be rescued, as strandings often happen in isolated areas. On some occasions, however, there have been successful efforts to refloat all the animals.

Mass stranding in Iceland, July 2019 © Svanhildur Egilsdottir/Marine & Freshwater Research Institute

Older mass stranding at Cape Cod.
Reproduction
Male pilot whales reach sexual maturity between 11 to 22 years of age (Desportes et al. 1993, 1994b), while females do so between the ages of 5 to 15. Both sexes reach physical maturity at age 25 to 30 (Bloch et al. 1993b, Martin and Rothery 1993). Gestation lasts about 12 months (Martin and Rothery 1993), with a significant level of foetal mortality (Desportes et al. 1994a). The fertility of females declines with age and whales older than 40 years rarely become pregnant (Martin and Rothery 1993, ICES 1996), although this not as systematic as in short-finned pilot whales (Foote 2008).
The birth interval was estimated to be 5.1 and 4.5 years off the Faroe Islands and in the Strait of Gibraltar respectively, the latter only including calves surviving the first year of life. The number of birth peaks in summer and autumn off the Faroe Islands (Martin and Rothery 1993) and in spring in the Strait of Gibraltar (Verborgh 2015), although newborn individuals are observed all year round.
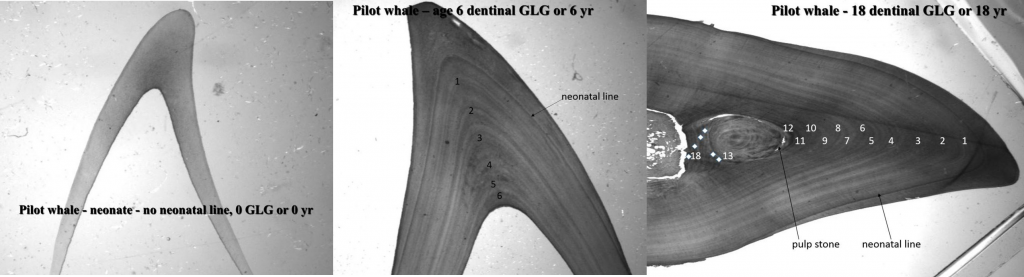
Growth layer groups in the dentin of three pilot whales aged 0, 6 and 18 years. © Christina Lockyer.
The mean size at birth is 178 cm and 75 kg. Although remains of prey, together with milk, are observed in calves only a few months old, young continue to nurse for 3–4 years on average, and nursing can last up to 7 years in males and 12 years in females. The duration of the lactation increases in older Faroese-caught females (Martin and Rothery 1993). This protracted lactation period is believed to be of social rather than nutritional importance allowing for long-lasting mother-calf bonds (Desportes and Mouritsen 1993). It is consistent with the stable matrilineal school structure described for the species (Andersen 1990, 1993, Amos et al. 1991, 1993a,b). Older and post-reproductive females possibly invest in their last calves, thus increasing the calves’ chance of survival by continuing to lactate and nurse. This in turn increase the likelihood of their genes being passed further.
Verborgh and Desportes (2021) reviewed survival rates in long-finned pilot whales and concluded that those estimated in the Mediterranean and off Newfoundland are within the range of those estimated off the Faroe Islands as 0.945 for males and 0.969 for females (Bloch et al. 1993a), but with first year calves showing a greater chance of survival off the Faroe Islands.
Feeding
Pilot whales primarily consume squid (and are thus called teuthophagous) and are essentially oceanic and deep-water feeders. However, they are opportunistic and exploit any locally abundant prey, in both the oceanic and neritic (coastal) habitats.
The relatively low number of teeth present in pilot whales (also found in other teuthophagous species such as Risso’s dolphins and sperm whales) is thought to be an evolutionary trait related to this diet consisting mainly of squids (Olson 2018).
Area specific
Off the Faroe Islands, pilot whales are clearly squid specialists and feed mainly on the European flying squid (Todarodes sagittatus) and the harmhook squid (Gonatus spp). Fish such as greater argentine (Argentina silus), blue whiting (Micromesistius poutassou), Greenland halibut (Reinhardtius hippoglossoides) and pandalid shrimps are also consumed. Although cod (Gadus morhua), Atlantic herring (Clupea harengus) and mackerel (Scomber scombrus) are common species in the Faroese neritic zone and have been reported as pilot whale prey elsewhere, they do not appear in the diet (Desportes 1985, Desportes and Mouritsen 1993).
Off Scotland, the French Atlantic coast, the NW Iberian Peninsula and the Mediterranean, the main component of the diet is also cephalopods, although fish are also present in varying degrees of importance, and the main prey varies between areas. Scottish whales consume oceanic squid species, while the benthic octopods constitute the most numerous prey off the Iberian Peninsula and France (Martin et al. 1987, Gonzáles et al. 1994, Pierrepont et al. 2005, Spitz et al. 2011, Praca et al. 2011, Santos et al. 2014). In the Mediterranean, pilot whales are also primarily teutophagus (Verborgh and Desportes 2021).
In the Northwest Atlantic, the short and long-finned squids Illex illecebrosus and Loligo pealei dominate the diet of pilot whales. The Atlantic mackerel, Atlantic herring and cod (Gadus morhua) have also been documented as present in the diet (Sergeant 1962, Mercer 1975, Overholtz and Waring 1991, Abend and Smith 1997, Gannon et al. 1997a,b, 1997b).
Adaptable
Pilot whales can thus feed on a wide variety of prey and consume both oceanic and neritic species as well as pelagic and benthic species. They adjust their diet in response to changes in prey abundance. Off the Faroes, in years where Todarodes is abundant on the Faroe shelf, the diet becomes nearly mono-specific, centred upon that species. In years where Todarodes is not abundant, the diet is more diverse and other species of squid and fish are consumed, species that were also abundant in Todarodes years but not preyed upon. They may feed predominantly on fish when squid are not readily available (Desportes and Mouritsen 1993).
This dietary plasticity allows pilot whales to forage in, and successfully occupy or shift to and from, oceanic and neritic habitats (Desportes 1985, Desportes and Mouritsen 1993). This the ability to exploit very different habitats is present in all areas (Spitz et al. 2011, Santos et al.2014). The use of ecological tracers reflecting longer-term diet, such as cadmium, carbon and nitrogen isotope ratios, and fatty acid signatures have also confirmed this foraging plasticity (Méndez-Fernandez et al. 2012, 2013, Monteiro et al. 2015a).
Distribution and Habitat
Long-finned pilot whales have an anti-tropical distribution in temperate and subpolar zones of the Northern and Southern Hemispheres. In the Northern Hemisphere, this species is found only in the North Atlantic Ocean, including the North Sea, Mediterranean Sea, and the Gulf of St. Lawrence (Minton et al. 2018). It has a typical temperature range of 0–25° C.
Long-finned pilot whales also occur off northeastern USA, but there they co-occur with short-finned pilot whales (Globicephala macrorhynchus), and the 2 species are difficult to discriminate at sea.
Distribution in the North Atlantic
Long-finned pilot whales are very widely distributed in the North Atlantic, from about 35–65° N in the west and from about 17–75° N in the east (Verborgh and Desportes 2021).
In the NAMMCO area, during summer months pilot whales are common in polar and subpolar waters, from Disco Bay in western Greenland, into the Irminger Sea, south of Iceland and around the Faroe Islands. They are rare off East Greenland and are considered irregular seasonal migrants in the Greenland, Norwegian and Barents Seas, although significant sized pods are occasionally seen as far north as Bear Island situated at 74.30° N (Kovacs et al. 2009, Hansen et al. 2018, Pike et al. 2019a,b).
Pilot whales are frequently sighted and hunted off southwest Greenland (Hansen et al. 2018, Hansen and Heide-Jørgensen 2013) but rare off East Greenland with an apparent gap in distribution in the area south of Greenland. This area has not been surveyed extensively and the gap may be an artefact.
They occur to the east and southeast of the North Atlantic Sightings Surveys (NASS) area off Britain and Ireland in the Bay of Biscay and are present mainly in oceanic areas of high biological productivity near the continental slope and submarine canyons, especially in the Rockall Trough northwest of Ireland (Hammond et al. 2009, Laran et al. 2017, Rogan et al. 2017). They are present there in a lower density than in northern European Atlantic waters (Hammond et al. 2009). Pilot whales mostly occupy the western basin of the Mediterranean Sea (Verborgh and Desportes 2021). Off the east coasts of Canada and the US, they have a more coastal distribution than on the eastern side of the Atlantic (Lawson and Gosselin 2018, Roberts et al. 2016).

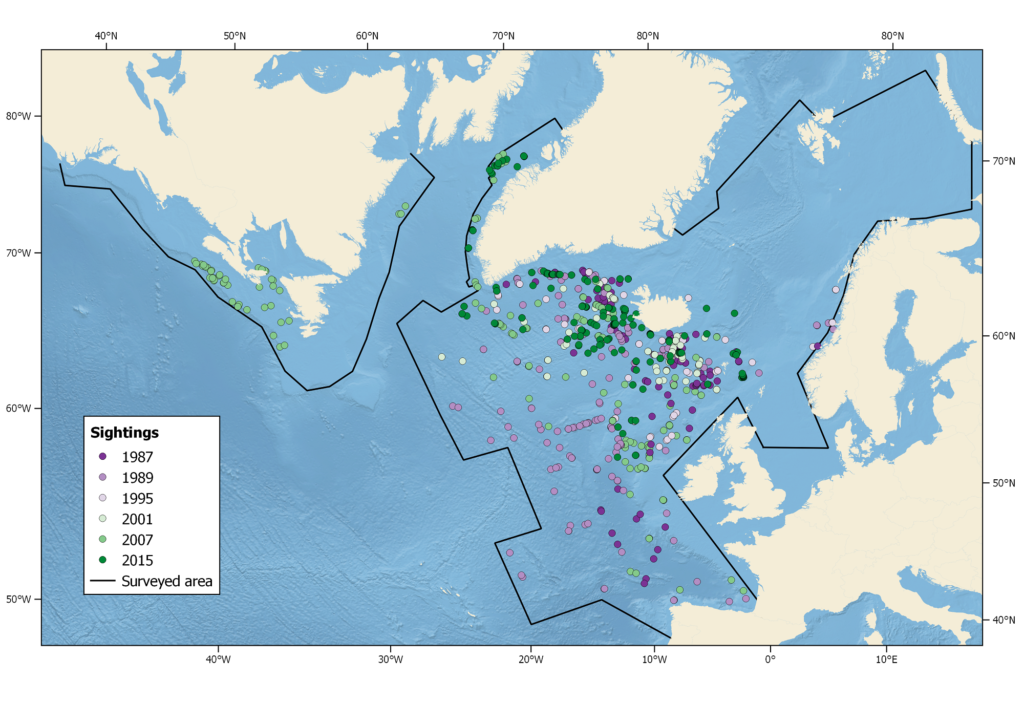
Summer distribution of long-finned pilot whales in the North Atlantic, showing sightings from all North Atlantic Sightings Surveys, 1987 – 2015, as well as 2007 CODA and SNESSA surveys.
Habitat use
Because of their high dietary plasticity, pilot whales can shift to and from oceanic and coastal habitats (see also the information given under the section Feeding). A recent spatial analysis of pilot whale distribution in the Northeast Atlantic (Rogan et al. 2017) indicated that summer numbers were highest in water depths >1,000 m and that they tended to be most abundant over areas of moderate slope. Abundance was also strongly associated with latitude, peaking at about 55° N and declining further north and south of this.
Fluctuations in movement and distribution
Pilot whale distribution does change from year to year, as is apparent from the distributions observed during the six NASS surveys (see sightings map). Although there is no indication that pilot whales undertake extensive seasonal migrations, their distribution also changes on a seasonal basis, with whales moving onto the slope, shelf and shelf edges over the summer months and moving back southwards into deeper offshore waters in winter (Sergeant and Fisher 1957, Sergeant 1962, Evans 1980, Payne and Heinemann 1993, Roberts et al. 2016). This is also reflected in the July–August peak in the frequency of pilot whale drives in the Faroes (Zachariassen 1993, Hoydal and Lastein 1993). Off western Ireland and the Bay of Biscay, however, seasonal variations are not apparent (Laran et al. 2017, Rogan et al. 2017).
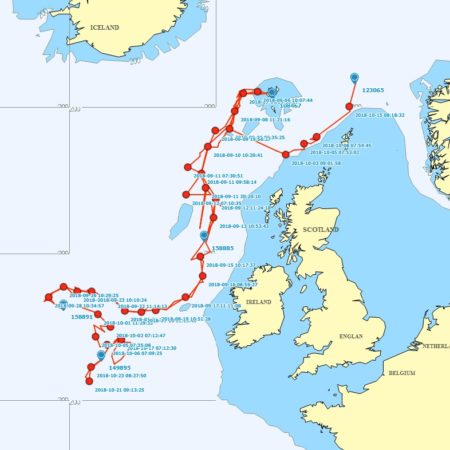
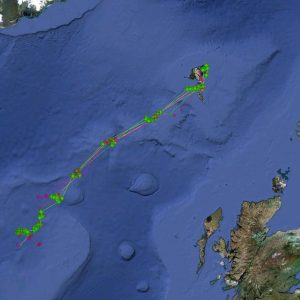
Satellite tagging of long-finned pilot whales off the Faroe Islands (Bloch et al. 2003, Mikkelsen pers. comm.) indicated that pilot whales can cover long distances in a relatively short time, with movements of up to 234 km recorded in one day and an average of 65–110 km/day. The tagged whales moved faster between apparent feeding/breeding areas where they would stay for several days or sometimes months. Some whales moved towards the southern Norwegian Sea while others predominantly towards eastern Iceland or south towards the Reykjanes Ridge (See the movement of different group of whales under the section Research done in NAMMCO Member Countries).
The abundance and movements of preferred prey seem to drive pilot whale abundance and movements in the North East Atlantic. This has been shown for flying squid off the Faroes (Desportes and Mouritsen 1993, Zachariassen 1993, Jákopsstovu 2002) and the northern shortfin squid off Newfoundland (Mercer 1975). The same pattern is observed off the United States and the Iberian Peninsula, with the longfin inshore squid and Atlantic mackerel and the northern shortfin and the lesser flying (Todaropsis eblanae) squids (Smith et al. 1990, Payne and Heinemann 1993, Gannon et al. 1997b, Santos et al. 2014).
North Atlantic Stocks
The stock structure of the North Atlantic population remains uncertain despite genetic, morphometric, physiological and observational studies (ICES 1993, 1996, Fullard et al. 2000, Oremus 2009, Monteiro et al. 2015b, Verborgh 2015).
Genetic studies
Mitochondrial DNA
Studies analysing differences in mitochondrial DNA (mtDNA) and allozymes in pilot whales across the North Atlantic have shown no significant differences between pilot whales from the western Atlantic, Iceland and the eastern Atlantic (Andersen 1993, Siemann 1994, Monteiro et al. 2015b). This could mean that there is only one stock of pilot whales in the North Atlantic.
However, this uniformity may be related to the social structure of pilot whales. If they occur in strong matrilineal (derived from the mother) schools, as suspected, then the actual population unit would be the school rather than the individual, making the genetically effective population size relatively small, in the order of several thousands, rather than hundreds of thousands (ICES 1996, NAMMCO 1998a). This small effective population might be expected to have a low mtDNA variability. Andersen (1993) found that schools captured in the Faroes differed in their allozyme composition, suggesting that genetic differences between schools do exist.
Microsatellite markers
More recently, a study using neutral microsatellite markers (Fullard et al. 2000) revealed significant genetic differentiation within the North Atlantic, and particularly between West Greenland and other both western and eastern regions (Cape Cod, Faroes and UK). This could not be explained by a simple isolation-by-distance model. Instead, the pattern of genetic differentiation suggested that stock separation occurs due to different prey type specialisation between areas of the ocean that differ in sea surface temperature, with 1) a cold-water population west of the Labrador/North Atlantic current, and 2) a warm-water population that extends across the Atlantic in the Gulf Stream.
Recently, Monteiro et al. (2015b) used both mitochondrial DNA (mitochondrial control region, neutral marker) and two loci of the Major Histocompatibility Complex (MHC, adaptive marker) on samples from the USA, Faroe Islands, Norway, the UK and Northwest Iberia to reveal a level of genetic substructure in the North Atlantic, with consistent divergence patterns describing Northwest Iberia as a separate group.
Recent studies, based on both microsatellite markers and mtDNA, indicate that the Mediterranean pilot whales are apparently isolated from Atlantic populations and that there may also be further structure within the Mediterranean basin (Verborgh 2015, Verborgh et al. 2016).
Non-genetic information

Movements of long-finned pilot whales satellite tagged in the Faroe Islands © Mikkelsen
More than two stocks in the North Atlantic
Two lines of evidence suggest that there are at least two stocks in the North Atlantic. There are morphometric (body shape) differences between pilot whales caught in the Northwest and Northeast Atlantic (Bloch and Lastein 1993, ICES 1996), indicating that pilot whales from these two areas are unlikely to be from the same stock. In addition, the depletion of pilot whales off Newfoundland from 1947 to 1972 apparently had no effect on pilot whale abundance elsewhere, indicating that there is probably little or no exchange between this area and others (Mercer 1975, Nelson and Lien 1996).
There may also be differences over smaller spatial and temporal scales. There were significant differences in pollutant concentrations (Aguilar et al. 1993, Caurant et al. 1993) and parasite burdens (Balbuena et al. 1993, 1994, 1995) between schools of whales landed in the Faroe Islands at different times and locations. This suggested that these schools had spent different proportions of their time, and fed, in different areas. This also pointed to stock differences and negated the hypothesis of just one resident Faroese population (ICES 1993).
Outlook
In 1997, the Scientific Committee of NAMMCO concluded that, based on some of the evidence noted above, it was likely that there was more than one stock of pilot whales in the North Atlantic, and more than one stock subject to the Faroese hunt (NAMMCO 1998a). It was apparent that further research was still required to resolve the stock delineation of pilot whales in the North Atlantic and this is still the case.
New genetic information strongly points to the existence of sub-structuring in the North Atlantic and information from 2011, 2012 and 2019 satellite tagging efforts clearly supports the conclusion that pilot whales caught in the Faroe Islands do not belong to a resident population.
Current Abundance and Trends
Estimating the abundance of pilot whales in the North Atlantic, as for other species of whales, has been largely based on sightings surveys conducted in summer from ships and airplanes. The North Atlantic Sightings Surveys (NASS) provide a time-series of abundance estimates from 1987 to 2015, covering a large part of the North Atlantic, and including Canadian waters in 2007 (Lawson and Gosselin 2009). Norwegian “mosaic” surveys cover most of the Northeast Atlantic, surveying a portion of the area annually on a 6-year rotation. In addition, the European CODA (Cetacean Offshore Distribution and Abundance in the European Atlantic, Hammond et al. 2009), the Canadian NAISS (Northwest Atlantic International Sightings Survey, Lawson and Gosselin 2018) and American SNESSA (Southern New England to Scotian Shelf Abundance, Palka 2020) surveys have contributed to our knowledge of pilot whale abundance and distribution.
Abundance in the North East Atlantic
The six NASS covered substantially different areas and, not surprisingly, yielded different population estimates for pilot whales (Pike et al 2019a,b, 2020). Year-to-year shifts in distribution are also apparent. The 1989 survey, conducted about 2 weeks later in the summer than the other surveys, extended farther south to cover the largest area of potential pilot whale habitat (1,279,741 nm²) from the west of Iceland to the Norwegian and Barents Seas north to Novaya Zemlya and south to the Bay of Biscay and its offshore waters to 25° W and 42° N (Buckland et al. 1993). It yielded the most reliable total estimate for the northeastern Atlantic of 778,000 (CV=0.295). No sightings were made in the north and northeast of the survey area, but sightings were made close to its southern border, indicating that the joint survey covered the northernmost areas of pilot whale distribution and that pilot whales also occurred south of the area surveyed.
The table below gives the abundance of pilot whales for the first five NASS, including for the Canadian area of T-NASS in 2007. The data for the Faroese and Icelandic areas were re-analysed to maintain consistency across surveys (Pike et al. 2013). These estimates differ slightly from published abundance estimates optimised for individual surveys (see NAMMCO 2020). An estimate from the NASS, SCANS and CODA areas combined from 2005–2007 is also presented, covering a very large area of the Northeast Atlantic.
The numbers suggest that the occupation or timing of occupation of the Northeast and Central Atlantic by pilot whales varies greatly from year to year. The 1989 estimate for the Iceland-Faroe Islands area is significantly higher than the 1987, 2001, 2007 and 2015 estimates, but, as mentioned above, the 1989 survey had a substantially different timing and coverage (larger area, extending more to the south and west) than the others, with the possibility of more pilot whales having moved inside the area.
Abundance of pilot whales from the NASS, CODA and SCANS.
CA, Canada; WG, West Greenland; EG, East Greenland; IF, Iceland – Faroe Island survey area; Per., perception bias; Avail., availability bias; y, corrected; n, uncorrected; p, partially corrected; CI, confidence interval; 1Confidence interval estimated assuming log-normal distribution and degrees of freedom > 30; 2Includes Faroese strata from T-NASS 2007.| Areas | Year | Area (NM^2) | Effort (NM) | Corrections per./avail. | Abundance | 95%CI | Source |
|---|---|---|---|---|---|---|---|
| NASS CA | 2007 | 337,109 | 25,274 | y/y | 32,999 | 14,468–75,265 | Lawson and Gosselin (2018)1 |
| NASS WG | 2007 | 58,573 | 2,093 | y/y | 8,133 | 3,765–17,565 | Hansen and Heide-Jørgensen (2013) |
| 2015 | 64,421 | 3,714 | y/y | 9,180 | 3,635–23,234 | Hansen et al. (2018) | |
| NASS EG | 2015 | 33,459 | 1,889 | y/y | 258 | 50–1,354 | |
| NASS IF | 1987 | 667,349 | 14,968 | n/n | 118,459 | 68,189–205,791 | Pike et al. (2013) |
| 1989 | 874,659 | 8,093 | n/n | 553,389 | 354,246–864,482 | ||
| 1995 | 709,194 | 6,182 | n/n | 226,665 | 125,630–408,956 | ||
| 2001 | 799,754 | 8,058 | n/n | 95,056 | 46,549–194,110 | ||
| 2007 | 750,410 | 5,875 | n/n | 128,093 | 75,682–213,802 | ||
| 2015 | 812,602 | 5,436 | y/n | 344,148 | 162,795–727,527 | Pike et al. (2019b) | |
| CODA + SCANS1 | 2005–07 | 881,588 | 25,502 | y/n | 172,195 | 88,194–336,206 | Rogan et al. (2017) |
| CODA + SCANS + TNASS | 2005–07 | 1,433,368 | 30,002 | p/n | 259,265 | 160,868–417,846 | Pike et al. (2013), Rogan et al. (2017)2 |
Trends in abundance
Off West Greenland, only 2 abundance estimates are available. The 2007 and 2015 NASS produced abundance estimates of similar magnitude, indicative of a stable population (Hansen et al. 2018) for this 8-year period.
The estimates listed above for the Iceland-Faroese area are not directly comparable to one another because the area covered is not the same for all surveys. However, Pike et al. (2019a) restricted the estimates to the area covered by all six surveys, and to the area covered by the three larger surveys (1989, 1995 and 2007). Because they cover exactly the same area, these “Survey Index Regions” are directly comparable between surveys.
The Survey Index Region covered by all six surveys shows a general decline in numbers from 1987 to 2007, followed by a recovery to the highest numbers yet observed in 2015. The Index Region covered by the three largest surveys shows a general decline from 1989 to 2007. However, the estimate from the smaller six-survey Index Region in 2015 is higher than most of those from the larger three-survey Index Region, indicating that a recovery would have been observed had the larger area been surveyed in 2015. This analysis therefore provides no evidence of any trend in pilot whale numbers in the Northeast Atlantic over the 28-year period from 1987 to 2015. The high level of variation in numbers between surveys within the Index Regions again confirms that the distribution of pilot whales differs greatly from year to year.
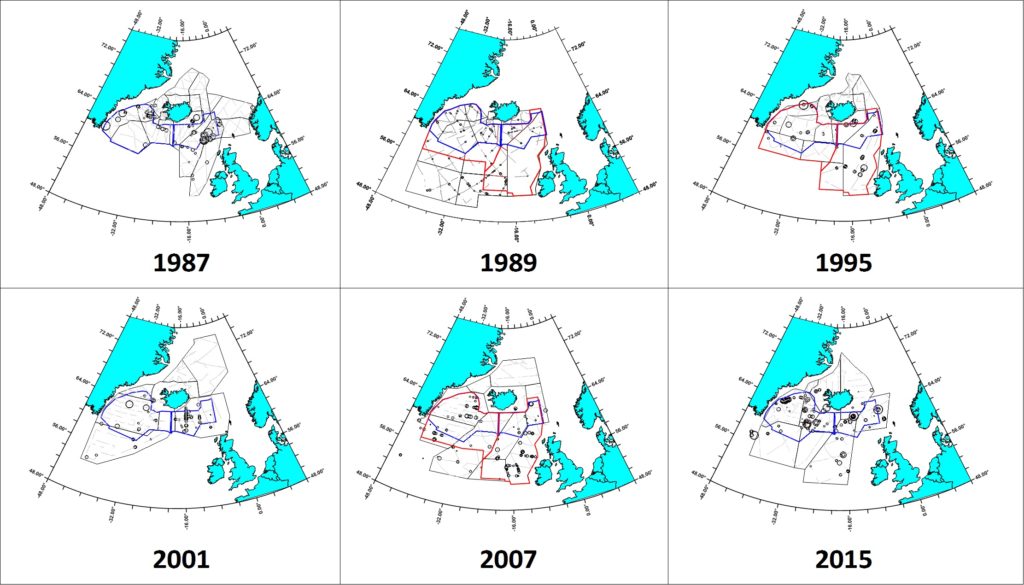
Pilot whale distribution in the Faroese and Icelandic components of the NASS. The six- and three- Survey Index Regions are outlined in blue and red, respectively.
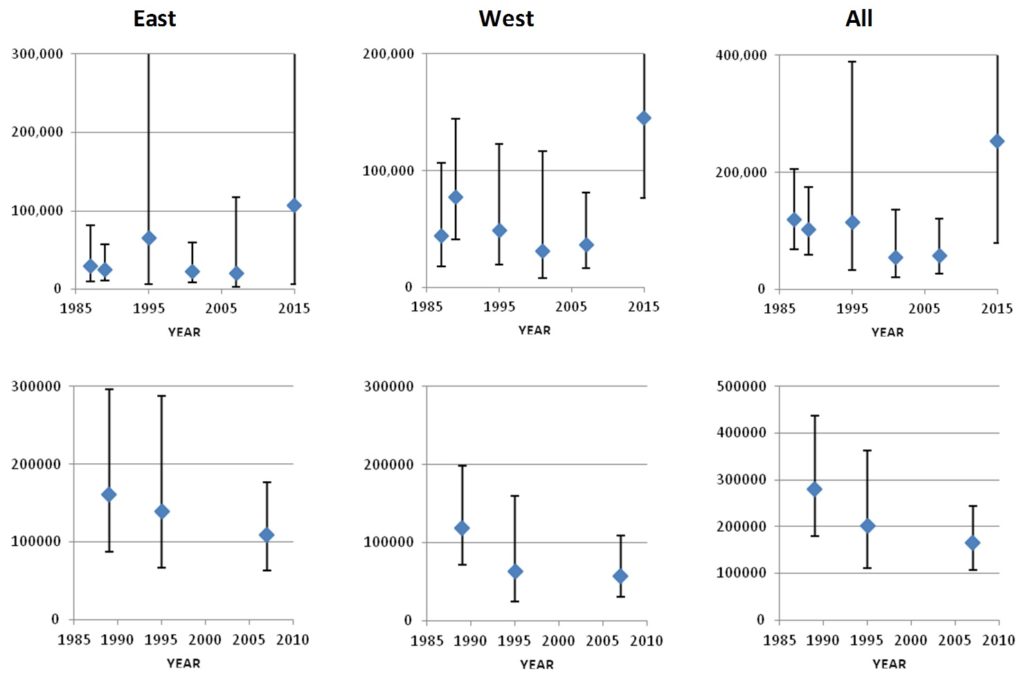
Trends in pilot whale abundance in the East, West, and Total areas of the Six Survey Index Region (top row) and the Three Survey Index Region (lower row).
Historic trends
An indication of long-term historical trends in the abundance of pilot whales around the Faroe Islands can be provided by an analysis of catch data. Catch records from the Faroes go as far back as 1584 and are unbroken since 1709 (Bloch 1994). Catch, corrected for hunting effort, shows a cyclic pattern with a period of 100–120 years, with peaks in catch occurring in 1720–1730, 1840–1850, and 1935–1985 (Hoydal and Lastein 1993). There is no long-term indication of declining or increasing abundance over the period (Bloch and Lastein 1995). The local availability of pilot whales to the Faroese may be related to changes in sea temperature and the abundance of their favoured prey. See under Life history and Ecology‘ for further details.

Pilot whales in the North Atlantic. © Nordlysid
Abundance and trends off the eastern U.S. and Canadian Atlantic coast
The table below provides the most recent data on distribution and abundance in the whole North Atlantic. Taken together, these estimates suggest that a population of well over 500,000 pilot whales occupies the North Atlantic area in some years. The population of the Western Atlantic, estimated to be over 40,000 pilot whales, is the smallest. This must be considered a minimum estimate because some areas where long-finned pilot whales are known to occur, such as Davis Strait and the Labrador Sea, and the offshore waters of the northeastern USA, remain poorly covered or completely unsurveyed. Also, most estimates come from ship surveys that are not corrected for whales that are submerged during the passage of the ship and therefore missed by the observers (Pike et al. 2019b).
Recent information on long-finned pilot whale abundance in the North Atlantic
(CI, confidence interval; CV, coefficient of variation; Corr., estimated corrected for; a, availability bias; p, perception bias).
| Area | Survey | Estimate | 95% CI | Corr. | Reference |
| Eastern USA | Central Virginia to Lower Bay of Fundy 2016 | 10,997 | (CV=0.51) | p | Hayes et al. (2020) |
| Eastern Canada | NAISS 2016 | 28,218 | 14,236–55,934 | a, p | Lawson and Gosselin (2018) |
| West Greenland | NASS 2015 | 9,180 | 3,635–23,234 | a, p | Hansen et al. (2018) |
| Barents Sea
Norwegian Sea |
NASSs/NILS 1987–2018 | Too few sightings to derive an abundance estimate | Øien pers. commn. | ||
| East Greenland | NASS 2015 | 258 | 50–1,354 | a, p | Hansen et al. (2018) |
| Iceland-Faroe Islands | NASS 2015 | 344,148 | 162,795–727,527 | p | Pike et al. (2019a) |
| Ireland maritime area | ObSERVE 2016 | 7,413 | 3,477–15,804 | a, p | Rogan et al. (2018) |
| Irish Sea | SCANSII 2005, CODA 2007, SCANSIII 2016, ObSERVE 2015–16 | No sightings | Hammond et al. (2017), Rogan et al. (2017, 2018) | ||
| North Sea & English Channel | NASSs/NILSs, SCANSII 2005, French 2011–2012, SCANSIII 2016 | No sightings | Øien pers. comm.
Laran et al. (2017), Rogan et al. (2017) |
||
| European continental shelf | 0207 CODA/FR TNASS
(covering thus a larger area than in 2016 |
172,195 | 88,194–336,206 | p | Rogan et al. (2017) |
| SCANSIII 2016 | 25,777 | 13,350–49,772 | a*, p | Hammond et al. (2017) | |
The species is listed as “Least Concern” on the global IUCN Red List, as it has a relatively wide distribution in temperate and subpolar waters of the Southern Hemisphere and in the North Atlantic and is common in some areas, although information on abundance and trends at the global scale is lacking. The total abundance estimate is approximately one million individuals, but large parts of the species’ range in the Southern Hemisphere have not been surveyed and therefore actual abundance is likely to be considerably greater than this. (Minton et al. 2018).
This status review focuses on the stocks within the remit of NAMMCO management advice and bordering populations. Verborg and Desportes (2021) provides the latest overall review of the long-finned pilot whale populations in European waters.
Greenland
Increasing numbers of long-finned pilot whales have been caught in recent years, particularly in East Greenland where the species was seldom caught before 2015. The annual catch of long-finned pilot whales in West Greenland during 1995–2018 averaged 217 whales.
The aerial NASS 2007 and 2015 surveys off West Greenland yielded abundance estimates of 8,133 pilot whales (95% CI: 3,765–17,565) and 9,180 pilot whales (95% CI: 3,635–23,234). The 2015 survey only partly covered the potential pilot whale habitat, as aggregations of pilot whales were encountered at the western edge of the survey area (Hansen and Heide-Jørgensen 2013, Hansen et al. 2018).
The NAMMCO Scientific Committee (2013) estimated that a sustainable catch level of pilot whales taken from the 2007 abundance would be between 50–70 whales per year. However, it seems likely that the size of the stock subject to the Greenlandic hunt is larger than that revealed by the survey, and that the summer aggregation off West Greenland cannot be considered an isolated stock. Instead, this stock is likely connected to pilot whales along Labrador and at Newfoundland, and the occurrence and abundance in West Greenland is probably influenced by the sea temperature regimes in the area (Fullard et al. 2000). For these reasons, the stock status of West Greenland pilot whales remains uncertain and needs to be monitored, although no decline has been detected between 2007 and 2015.
Eastern Atlantic
A trend analysis incorporating all six NASS surveys covering the 28-year period from 1987 to 2016 found no trend in pilot whale numbers over the period (Pike et al. 2019a). Given the minimum size of the population being over 500,000 animals (according to the most recent surveys) and likely well over 500,000 animals in the Northeast Atlantic (from the 1989 estimate), it seems very unlikely that an annual catch of around 1,000 whales in the period 1987–2015 would cause a reduction in the population. The fact that the population has been subject to approximately the same level of catch for at least 300 years, with apparently little change in availability (Hátún and Gaard 2010), also suggests that the Faroese catch is not likely to be causing the stock to decline. In 1997, NAMMCO concluded that the drive hunt was sustainable (NAMMCO 1998b).
Impact of the catch related to geographic range
The effect of catches of pilot whales in the Faroe Islands depends on the geographic range of the population which is affected by these catches (ICES 1996, see figure below). If the whales come from the Mid-Atlantic Ridge-Iceland Area (A+B+C) or the area covered by the NASS 1989 (A+B+C+D) sighting survey, then catches over the last 150 years have hardly had any impact on the population trajectory. However, the qualitative effect of the catches would be very different if they were coming from a population with a geographic range the size of the Rockall-Iceland Area (A+B) or the Faroese Islands Area (A).
Although the stock delineation of pilot whales remains unclear, non-genetic evidence – pollutant concentrations and parasite burdens, indicates that there is likely more than one stock subject to the Faroese hunt (See under North Atlantic Stocks, ICES 1996, NAMMCO 1998a) and that this hunt is not targeting a small resident population. The satellite tagging data also shows that pilot whales caught off the Faroes moved well beyond the Faroe Islands Area. Indeed, the satellite tagging data from 2012 and later years revealed whales moving. 3-NASS Survey Eastern Index Region.
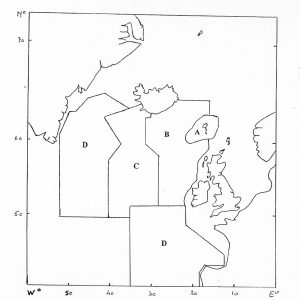
Sustainability
The abundance of pilot whales required to sustain an annual catch of 678 animals (the average annual Faroese catch 1997–2011) is between 50,000 and 80,000 whales (with a precision equivalent to that of the sightings survey in 2007) (NAMMCO 2013). The population in the Eastern Index area, which is smaller than the range of the population affected by the catch, is alone over 100,000 pilot whales.
That being said, the last formal assessment of the species (ICES 1996) is now several decades old. The NAMMCO Scientific Committee is planning a new assessment tentatively scheduled for 2022, which will take into account all new information, including new estimates of abundance and trend analyses.
Status of stocks in bordering areas
US Atlantic
In 1999, Waring et al. (1999) concluded that although there was no information on abundance trends, abundance is likely increasing because human-induced mortality had declined in recent years.
The 2019 US assessment (Hayes et al. 2020) concluded that the status of long-finned pilot whales in the U.S. Atlantic EEZ was unknown. There was insufficient data to determine population trends for this species. The total U.S. fishery-related mortality and serious injury for long-finned pilot whales is less than 10% of the calculated PBR (Potential Biological Removal) and, therefore, can be considered insignificant and approaching zero mortality and serious injury rate. The long-finned pilot whale is not listed as threatened or endangered under the Endangered Species Act, and the western North Atlantic stock is not considered strategic under the MMPA because the mean annual human-caused mortality and serious injury does not exceed PBR.
Canadian Atlantic
Pilot whales were subject to an intensive drive fishery in Newfoundland from 1947 to 1972, and this fishery apparently reduced the stock to very low levels (Mercer 1975, Hay 1982, Nelson and Lien 1996). Abundance at the onset of the fishery was likely around 60,000 animals (Mercer 1975). In 1980, Hay (1982) conducted an aerial survey in eastern Newfoundland and southeastern Labrador waters, and estimated 13,167 whales (95% CI: 6,731–19,602, not corrected for diving whales or whales missed by observers). Nelson and Lien (1996) concluded that the population had likely not yet recovered to its pre-exploitation size.
This seems to have been confirmed by the results of the 2016 Canadian NAISS survey (Lawson and Gosselin 2018), which provided a full coverage of the Atlantic Canadian coast and yielded a fully corrected abundance estimate of 28,218 (approximate 95% CI: 14,236–55,934). The estimate from the Canadian component of the 2007 T-NASS survey (32,999 pilot whales, 95% CI: 14,468–75,265) was of a similar magnitude (Lawson and Gosselin 2018). Taken together, these surveys suggest that pilot whale numbers are stable or recovering in the area.
Outlook
Pilot whales are likely one of the most abundant odontocetes in the North Atlantic. The hunt of pilot whales that continues in the Faroe Islands today has proven sustainable over a period of more than 400 years. In 1997, NAMMCO concluded that the drive hunt was sustainable (NAMMCO 1998b). The population off Newfoundland is likely slowly recovering after a period of excessive hunting.
In some other areas, by-catch of pilot whales in fisheries continues, although apparently at relatively low levels. While there is at present no direct indication that pilot whales are being affected by contaminants, their occupation of a relatively high trophic level and the high level of contaminants observed does make them susceptible to this threat (AMAP 2018a,b).
Better information on the abundance and trends in abundance of pilot whales is required to ensure the long-term sustainability of the present removals. In addition, further information on stock delineation and geographical range of stock components would improve the ability to design and carry out effective surveys for this species. In light of new genetic information, the recent tagging data, as well as the new abundance estimates obtained from the 2015 NASS survey and the 2016 SCANS III survey, a new assessment is warranted. NAMMCO plans to conduct a full assessment of the species in 2022.

North Atlantic long-finned pilot whales. © IMR, Norway
Status according to other International Organisations
CITES – Convention on International Trade in Endangered Species of Wild Fauna and Flora
Long-finned pilot whales are currently listed on Appendix II of the CITES. CITES is a legally binding multilateral environmental agreement that aims to ensure that international trade does not threaten the survival of species in the wild. Both Greenland and the Faroe Islands are signatories to the convention through Denmark. A listing on Appendix II means that an export permit shall only be granted when the Scientific Authority of the State of export has advised that such export will not be detrimental to the survival of the species in the wild. There is, however, no export of pilot whale products from Greenland or the Faroe Islands, except for a very small non-commercial export of meat and blubber for family consumption.
Long-finned pilot whales inhabit the waters of the four NAMMCO member countries: Faroe Islands, Greenland, Iceland and Norway. They are protected in Iceland and Norway.
Greenland
Pilot whales are taken on an opportunistic basis (a few hundred a year, see under Recent Catches), mainly in southwest Greenland but since 2015 also regularly in East Greenland, though in lower numbers. There are no quotas for pilot whales in Greenland, however there are regulations pertaining to hunting methods and equipment.
Iceland
The long-finned pilot whale is protected in Iceland. When mass strandings occur, the latest in 2019 (see under Mass Strandings), the authorities and the Marine and Freshwater Research Institute deal with the event and any collection of biological samples from the animals.
Faroe Islands
The Faroese pilot whale hunt is regulated by legislation that deals with all aspects of the hunt in detail, including driving procedures, beaching, killing methods, obligatory training of hunters, and approved equipment, valuation, distribution and beach clean-up (e.g., Joensen 2009, Anonymous 2013). These regulations are subject to regular review and have been updated and revised a number of times in recent years, both to keep them consistent with technological developments and to refine some of the organisational aspects of the drive and the distribution.
Contrary to the situation in Newfoundland, where the pilot whale stock became severely depleted by the commercial hunt which provided whale meat to the province’s mink and fox fur farms, pilot whaling in the Faroe Islands is forbidden by law from becoming commercial. The management system is designed to provide food for the residents while not depleting the resource through unnecessary extraction (Fielding 2010).
The Drive
A recently developed spinal lance has been introduced as mandatory for the killing of pilot whales. The spinal lance has been shown to reduce killing time to 1–2 seconds, while also improving accuracy and safety (Anonymous 2013). A blowhole hook has also been developed. Since May 2015, only people that have attended a certified course in the whaling regulations and killing methods are permitted to kill whales. NAMMCO published an Instruction Manual on Pilot Whaling in 2014, and the manual exists in both Faroese and English.
Only specific bays that meet the requirements for suitable whale beaching conditions are allowed to be used for the drive (today there are 23 such bays across the Faroe Islands,). The hunt is supervised by elected grind foremen, who are themselves under the supervision of the sýslumaður, or district sheriff. The sýslumaður also oversees the valuation and division of the catch and is responsible for keeping the records of the catch and the distribution (Bloch et al. 1990, Bloch 2007, Joensen 2009, Anonymous 2013).
There are no quotas, but beaches or entire whaling districts are closed when the supply of meat is considered sufficient by the authorities.
Division of the Catch
The catch has always been shared among those taking part in the drive together with the local residents of the whaling bay and district in accordance with a complex, traditional community sharing system. The division of the catch is administered by the relevant sýslumaður. The catch is divided into shares known in Faroese as a skinn, which is an age-old measurement value that derives from agricultural practices. One skinn is roughly equivalent to 34 kilograms of blubber and 38 kilograms of meat. Whales in the catch are numbered, their condition is assessed, and they are valued using a traditional wooden pole calibrated in logarithmic division representing the value in skinn. Their value in skinn is marked in roman numerals on the flipper (Joensen 1976, 2009, Bloch et al. 1990, Bloch 2007).
Consumer Health
“Eat whale meat and blubber, then you will grow tall and strong,” is an old Faroese saying. Pilot whale meat and blubber contain many valuable nutrients but today also contain high levels of contaminants. Pilot whales live high up in the food chain and accumulate higher levels of pollutants than many other marine resources. The levels of PCBs and mercury are relatively high in the blubber and meat of Faroese-caught pilot whales (see under Pollution). Given that pilot whale meat and innards have been a staple food in the islands, the Faroese Health Authorities have since 1998 been providing regular recommendations on the consumption of pilot whale meat and blubber based on the latest research and internationally applied standards for precautionary limits. The latest recommendations were given in June 2011.
You can find more information on the Faroese pilot whaling here, as well as accessing the last executive order on the pilot whale drive (July 2013) in English translation.






Norway
Pilot whales are protected in Norway, both in Svalbard and mainland Norway.
Management in bordering areas
As the population structure is not elucidated and stock delineations are not solved, the long-finned pilot whale population under the remit of NAMMCO management is considered to be shared with outside jurisdictions, such as Canada, the UK, and the EU.
Canada and USA
There is no fishery targeting pilot whales in Canada today. The Committee on the Status of Endangered Wildlife in Canada (COSEWIC) listed the long-finned pilot whale as not at risk in 1994. Neither (COSEWIC) nor the Species at Risk Act (SARA) presently consider the long-finned, or the short-finned, pilot whale at-risk of extinction.
Long-finned pilot whales, like all marine mammals, are protected throughout their range under the US Marine Mammal Protection Act.
United Kingdom
Pilot whales, as other cetacean species, are protected in the UK.
European Union
The ‘Habitats Directive’ (the 1992 Council Directive 92/43/EEC on the Conservation of Natural Habitats and of Wild Fauna and Flora) is the main policy driver for nature conservation in European waters. The pilot whale is listed under Annex IV (species of community interest in need of strict protection).
Hunting
Pilot whales have a long history of utilisation by humans in the North Atlantic. In the 20th century, they were caught in Greenland, Iceland, the Faroe Islands, western Ireland, the Shetland and Orkney Islands, Norway, eastern USA and Newfoundland in Canada (Mitchell 1975, Nelson and Lien 1996). They continue to be caught in the Faroe Islands and Greenland. In most places, pilot whaling was conducted as drive fisheries. Meat and blubber, and diverse organs, have been used for human consumption.
Greenland
Pilot whales are taken on an opportunistic basis, mostly in southwest Greenland. They are usually hunted from small boats using rifles and hand harpoons. In the period 2000–2018, annual catches have averaged 248 and ranged from 5 to 433 whales.
Faroe Islands

Grindadráp, S. Joensen-Mikines 1959. Listasavn (Museum of Art), Tórshavn, Faroe islands
Drive fisheries for pilot whales in the Faroe Islands—known in Faroese as grindadráp—date back to the Norse settlement in the 9th century, with written descriptions from as early as 1632 (Sanderson 1992). It is seen to be “a distinctive cultural characteristic for the Faroe Islands” (Joensen 1976) and “an established symbol of Faroese national identity” (Sanderson 1992). The grindadráp has inspired the production of extensive cultural material including literature, poetry, painting, sculpture, handicrafts, music and songs for centuries (Joensen 2009, Sanderson 1992). Whaling equipment is often displayed in houses as décor, taking it beyond its mere utilitarian purpose.
Catches
Catch statistics exist since 1584 and are unbroken from 1709 to today. They show an annual average catch of 850 pilot whales (range 0–4,480) with a cyclical variation correlated with North Atlantic climatic variations and oceanic events (Hoydal and Lastein 1993, Bloch and Lastein 1995, Block 1998, Jákupsstovu 2002, Hátún et al. 2009, Hátún and Gaard 2010).
Between 1709 and 1999, a total of 246,434 pilot whales were caught in 1,766 pods. There was an average of 6.1 grinds (whale drives) per year, and grind size ranged from 1 to 1200 whales, with a mean of 139.5 whales per grind (Zachariassen 1993, Bloch 1994). Since 2000, catches have ranged between 0 and 1203, with a yearly average for the period 2000–2020 of 632 animals (see table of reported catches below).
When whales are sighted, local small fishing vessels cooperate to drive the whales into designated beaches. The whale is secured with a blowhole hook, after which the spinal lance is positioned in the midline between the blowhole and the dorsal fin at one hand’s breadth behind the blowhole and directed at an angle approximately 10 degrees backward. With a single thrust followed by sideways movements the spinal cord and the surrounding blood vessels are severed, directly followed by severing the jugular and the carotid arteries with a whaling knife so that the whale can be bled properly. Once the cut is made, the whale lies completely paralysed, unconscious and dies.
Canada and USA
An important pilot whale drive fishery was active at Cape Cod from the mid-1700s to the 1920s. During the late 1800s, the mean annual catch was in the order of 2–3,000 animals. A drive fishery was also active off the shores of Virginia and North Caroline (Mitchell 1975 a,b).
Newfoundland’s drive fishery was active from 1947 to 1964 and at its highest in 1956, with catches reaching almost 10,000 animals (Sergeant 1962, Mitchell 1975a). It declined shortly after and is now defunct.
Utilisation
Pilot whale meat is high in protein (higher than beef) and low in fat. Because a whale’s fat is contained in the layer of blubber beneath the skin and the muscle is high in myoglobin, the meat has a dark red colour (Bloch 2007).
Greenland
Pilot whales are taken on an opportunistic basis and principally in southwest Greenland. They are used for human consumption.
Faroe Islands
In the Faroe Islands, hunting of pilot whales has been an important source of food for the inhabitants since the islands were colonized (e.g., Williamson 1970). The cultural connection to traditional local food remains strong (Fielding 2010) and most of the ancient hunting traditions are kept alive, including pilot whaling. The traditional forms of food production are a welcome contribution to the household economy. In 2002, pilot whaling supplied 30% of all locally produced meat.
Although of less economic significance today, the free sharing of pilot whale meat and blubber from the hunt has contributed to good health and to the survival of many elders, low-income families and families where men and boys were absent for weeks and months fishing, or simply disappeared at sea (Joensen 2009).
Both the meat and blubber of pilot whales have long been—and continue to be—a valued part of the national diet. Managed through the community as a common property resource (Kerins 2008), catches are shared largely without the exchange of money among the participants in a whale drive and residents of the local district where they are landed (e.g., Bloch 2007, Anonymous 2013). The persistence of this communal, non-commercial subsistence-based hunt for food in what is in other respects a modern technological Western society is a characteristic feature of the Faroese grind (Sanderson 1994).
On average, 54% of the total weight of a whale is consumed as meat and blubber, with this increasing to up to 60% in larger whales, which have proportionally larger muscle mass than smaller whales. In comparison, an average of 47% of fish weight is consumed (Bloch 2007).
Whale meat and blubber are stored, prepared and eaten in a variety of ways (Joensen 2009, Bloch 2007, Anonymous 2013). When fresh, the meat is boiled or served as steaks, with blubber and potatoes. The meat and blubber can be frozen or preserved using traditional Faroese methods such as dry-salting or storing in brine. Strips of whale meat are also hung to wind-dry for several weeks and then eaten raw in thin slices. Thin slivers of blubber are also a popular accompaniment to dried fish. Boiled whale meat and blubber, served with potatoes and mustard, is a standard family meal.



Did you know?
A pilot whale stomach can be used as a buoy
A pilot whale stomach can be used as a buoy. Pilot whale stomachs were used as buoys until the 1970s. Pilot whale stomachs have three compartments. The first one, called the mechanical stomach, is made of very strong tissues and acts as a grinder. To make a buoy, this part was emptied, placed in brine, then tanned and inflated for use on long lines (left). In earlier days, thongs of dried skin from the dorsal ridge were used in the boats to tie the oars to the wooden posts which serve as a rowlock (right).


Recent Catches
Greenland
Since 2000, there has been an average catch of around 250 whales per year, in a range from 5 to 433 (see table of reported catches below). Catches are mostly taken in West Greenland, where they have ranged between 5 and 433 animals per year. In East Greenland catches have ranged between 0 and 126 per year, with an annual average of 15 whales. Catches have increased in East Greenland, from being opportunistic until 2015 (from 0 to 20, per year), to an annual average of 73 whales in 2016–2018.
Faroe Islands
Since 2000, the annual pilot whale catches have ranged between 0 and 1203, with an average for 2000–2020 of 632 animals (see table of reported catches below).

Butchering pilot whales © FMNH
Catches in NAMMCO member countries since 1992
| Country | Species (common name) | Species (scientific name) | Year or Season | Area or Stock | Catch Total | Quota (if applicable) |
|---|---|---|---|---|---|---|
| Faroe Islands | Long-finned pilot whale | Globicephala melas | 2023 | Faroe Islands | 897 | |
| Faroe Islands | Long-finned pilot whale | Globicephala melas | 2022 | Faroe Islands | 528 | |
| Faroe Islands | Long-finned pilot whale | Globicephala melas | 2021 | Faroe Islands | 667 | |
| Faroe Islands | Long-finned pilot whale | Globicephala melas | 2020 | Faroe Islands | 530 | |
| Faroe Islands | Long-finned pilot whale | Globicephala melas | 2019 | Faroe Islands | 682 | |
| Faroe Islands | Long-finned pilot whale | Globicephala melas | 2018 | Faroe Islands | 624 | |
| Faroe Islands | Long-finned pilot whale | Globicephala melas | 2017 | Faroe Islands | 1203 | |
| Faroe Islands | Long-finned pilot whale | Globicephala melas | 2016 | Faroe Islands | 295 | |
| Faroe Islands | Long-finned pilot whale | Globicephala melas | 2015 | Faroe Islands | 501 | |
| Faroe Islands | Long-finned pilot whale | Globicephala melas | 2014 | Faroe Islands | 48 | |
| Faroe Islands | Long-finned pilot whale | Globicephala melas | 2013 | Faroe Islands | 1104 | |
| Faroe Islands | Long-finned pilot whale | Globicephala melas | 2012 | Faroe Islands | 713 | |
| Faroe Islands | Long-finned pilot whale | Globicephala melas | 2011 | Faroe Islands | 726 | |
| Faroe Islands | Long-finned pilot whale | Globicephala melas | 2010 | Faroe Islands | 1107 | |
| Faroe Islands | Long-finned pilot whale | Globicephala melas | 2009 | Faroe Islands | 310 | |
| Faroe Islands | Long-finned pilot whale | Globicephala melas | 2008 | Faroe Islands | 0 | |
| Faroe Islands | Long-finned pilot whale | Globicephala melas | 2007 | Faroe Islands | 633 | |
| Faroe Islands | Long-finned pilot whale | Globicephala melas | 2006 | Faroe Islands | 856 | |
| Faroe Islands | Long-finned pilot whale | Globicephala melas | 2005 | Faroe Islands | 302 | |
| Faroe Islands | Long-finned pilot whale | Globicephala melas | 2004 | Faroe Islands | 1012 | |
| Faroe Islands | Long-finned pilot whale | Globicephala melas | 2003 | Faroe Islands | 503 | |
| Faroe Islands | Long-finned pilot whale | Globicephala melas | 2002 | Faroe Islands | 626 | |
| Faroe Islands | Long-finned pilot whale | Globicephala melas | 2001 | Faroe Islands | 918 | |
| Faroe Islands | Long-finned pilot whale | Globicephala melas | 2000 | Faroe Islands | 588 | |
| Faroe Islands | Long-finned pilot whale | Globicephala melas | 1999 | Faroe Islands | 608 | |
| Faroe Islands | Long-finned pilot whale | Globicephala melas | 1998 | Faroe Islands | 815 | |
| Faroe Islands | Long-finned pilot whale | Globicephala melas | 1997 | Faroe Islands | 1162 | |
| Faroe Islands | Long-finned pilot whale | Globicephala melas | 1996 | Faroe Islands | 1556 | |
| Faroe Islands | Long-finned pilot whale | Globicephala melas | 1995 | Faroe Islands | 228 | |
| Faroe Islands | Long-finned pilot whale | Globicephala melas | 1994 | Faroe Islands | 1201 | |
| Faroe Islands | Long-finned pilot whale | Globicephala melas | 1993 | Faroe Islands | 808 | |
| Faroe Islands | Long-finned pilot whale | Globicephala melas | 1992 | Faroe Islands | 1572 | |
| Greenland | Long-finned pilot whale | Globicephala melas | 2023 | East | 46 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2023 | West | 82 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2023 | Total | 128 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2022 | Total | 92 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2021 | West | 135 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2021 | East | 61 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2020 | East | 117 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2020 | West | 208 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2020 | Total | 225 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2019 | East | 178 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2019 | West | 127 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2019 | Total | 305 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2018 | East | 136 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2018 | West | 263 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2018 | Total | 399 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2017 | East | 67 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2017 | West | 317 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2017 | Total | 384 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2016 | East | 27 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2016 | West | 168 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2016 | Total | 195 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2015 | East | 6 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2015 | West | 277 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2015 | Total | 283 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2014 | East | 0 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2014 | West | 433 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2014 | Total | 433 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2013 | East | 0 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2013 | West | 316 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2013 | Total | 316 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2012 | East | 6 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2012 | West | 426 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2012 | Total | 432 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2011 | East | 2 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2011 | West | 272 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2011 | Total | 274 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2010 | East | 0 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2010 | West | 338 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2010 | Total | 338 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2009 | East | 1 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2009 | West | 237 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2009 | Total | 238 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2008 | East | 0 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2008 | West | 182 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2008 | Total | 182 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2007 | East | 20 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2007 | West | 268 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2007 | Total | 288 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2006 | East | 0 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2006 | West | 46 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2006 | Total | 46 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2005 | East | 0 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2005 | West | 345 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2005 | Total | 345 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2004 | East | 9 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2004 | West | 256 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2004 | Total | 265 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2003 | East | 9 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2003 | West | 186 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2003 | Total | 195 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2002 | East | 15 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2002 | West | 23 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2002 | Total | 38 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2001 | East | 1 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2001 | West | 61 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2001 | Total | 62 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2000 | East | 0 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2000 | West | 5 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 2000 | Total | 5 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 1999 | East | 0 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 1999 | West | 115 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 1999 | Total | 115 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 1998 | East | 0 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 1998 | West | 365 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 1998 | Total | 365 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 1997 | East | 0 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 1997 | West | 232 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 1997 | Total | 232 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 1996 | East | 0 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 1996 | West | 71 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 1996 | Total | 71 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 1995 | East | 0 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 1995 | West | 1 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 1995 | Total | 1 | No quota |
| Greenland | Long-finned pilot whale | Globicephala melas | 1992-1994 | Total | *No reported catches | No quota |
This database of reported catches is searchable, which means that it is possible to filter the information by country, species or area. It is also possible to sort the information by the different columns, in ascending or descending. By default, 30 entries are shown, but this can be changed in the drop-down menu.
The full catch database with all species can be found here.
For any questions regarding the catch database, please contact the Secretariat at nammco-sec@nammco.no.
Anthropogenic activities which may affect marine mammals generally and pilot whales specifically can be divided into two main categories: habitat degradation and oceanographic changes. Knowledge about pilot whale habitat use is limited and it is therefore difficult to assess the relative impact that such changes may represent for the species.
Habitat degradation
A range of human activities have the potential to degrade habitat that is important for pilot whale survival. The species is essentially oceanic but also occurs in coastal waters. Main threats include:
- changing water quality and pollution (e.g., runoff from agriculture, oil spills, acidification)
- entanglement (e.g., in marine debris, ghost nets, etc.)
- prey depletion due to over-harvesting
- acoustic pollution with disturbance and/or masking from loud anthropogenic sounds, such as those generated by navy sonar and seismic exploration.
Pollution
In addition to their direct toxicity, anthropogenic contaminants may affect animal resilience and increase susceptibility to disease in marine mammals (Reijnders and de Ruiter-Dijkman 1995). The accumulation of contaminants in pilot whales has been extensively studied on both sides of the Atlantic (e.g., Aguillar et al. 1993, Borrell 1993, Borrell and Aguilar 1993, Borrell et al. 1995, Caurant et al. 1993, 1994, 1996, Caurant and Amiard-Triquet 1995, Tilbury et al. 1999, Dam and Bloch 2000, Weisbrod et al. 2000, 2001, Sonne et al. 2010, Méndez-Fernandez et al. 2014a,b, Hoydal et al. 2015, Bjurlid et al. 2018). Studies dating back to 1977 have shown an overall increase in contamination of the meat, blubber, liver and kidneys of pilot whales. Together with the killer whale, the long-finned pilot whale is one of the most polluted species in the world (AMAP 2004, 2005, 2018a,b, Dam and Bloch 2000).

Faroese stamp issued in 2010.
Organic contaminants
Faroese pilot whales have concentrations of organic contaminants in their blubber that are roughly in the mid-range of such concentrations in other species of toothed whales in the North Atlantic (Borrell and Aguilar 1993, Mendez-Fernandez et al. 2014b). It is not known if these contaminants are affecting the health of pilot whales in the North Atlantic, but their role in the high mortality of pilot whales observed during the 2006-07 morbillivirus epizootic in the Mediterranean has been questioned (Lauriano et al. 2014). These types of contaminants would be transferred to the offspring during lactation (Borrell et al. 1995), which could decrease the chances of survival for the calves (Hoydal et al. 2015).
In the Faroe Islands, flame retardants (PBDEs) have been documented in pilot whales at above the threshold levels associated with endocrine disruption. However, their concentrations in juvenile males from the Faroe Islands have decreased between 1996 and 2013 (Bjurlid et al. 2018).
Monitoring results indicate a steadily decreasing concentration of persistent organic pollutants (POPs) (i.e., PCBs and legacy pesticides, including brominated flame retardants PBDE) in general in the marine environment, while for perfluorinated alkyl substances, there is no clearly discernible trend, although concentrations appear to have levelled out or be on a weakly increasing curve (Andreasen et al. 2019).
Trace and heavy metals
The concentration of trace metals (other than copper) in the liver of pilot whales from the Faroe Islands is at the higher end of the range of what is found in other marine mammal species of the North Atlantic (Caurant et al. 1993) and pilot whales from other area of the North Atlantic (Méndez-Fernandez et al. 2014a). Cadmium levels, in particular, are very high and even higher than in species from highly industrialized areas (Caurant et al. 1993). This is probably related to their heavy consumption of squids (Bustamante et al. 1998).
The heavy metals mercury (Hg) and cadmium (Cd) produce adverse effects in various mammals, with risks of health impacts on internal organs, such as the liver and kidneys. Possible direct and indirect effects (e.g., immunodeficiency) of trace metals on pilot whales have been little studied. However, a recent study showed a high prevalence of histopathological changes in liver and renal tissues of Faroe Island pilot whales and suggested that age, heavy metal, and organic contaminants may be important factors in pathology development (Sonne et al. 2010).
The Arctic Monitoring and Assessment Programme (AMAP) most recent assessments of trends in Arctic contaminants and their effects on wildlife and fish show that, while international and national pollution control activities have generally been effective at reducing the levels and ecosystem impacts of the chemicals they regulate, some contaminants including PCBs and mercury continue to pose a significant risk to some Arctic biota. In particular, top predators including pilot whales are at continuing risk from exposure to these contaminants. In addition, as new chemicals of Arctic concern enter into use in society, the suite of environmental contaminants found in Arctic top predators is expanding (AMAP 2018a,b).
Consumer health
The Faroe Islanders rely on pilot whales as a local wildlife food resource. In some cases, the level of consumption of pilot whale meat and blubber by Faroese may lead to an intake of these substances that exceeds recommended levels. This is of particular concern given the documented neurotoxicity of methylmercury, in particular to the developing foetus (Weihe et al. 1996, for more details see under Contaminants). The consumption of pilot whale meat is thought to represent a hazard to the health of consumers (Weihe et al. 1996, Steuerwald et al. 2000, Weihe and Joensen 2008, 2012, Choi et al. 2009, Grandjean et al. 2011). Consequently, since 1998 the Faroese health authorities have provided regular recommendations regarding the consumption of pilot whale meat and blubber, in response to the latest research and based on internationally applied standards for precautionary limits. The most recent recommendations were given in in June 2011. Weihe and Joensen 2012 recommended that pilot whales be no longer used for human consumption.
As more developing nations industrialise, marine pollution is likely to increase. As pilot whale meat and blubber become more contaminated, the risk of its consumption may come to outweigh the benefits (Fielding 2010). If pilot whale hunting were to cease, it would likely not be because of over-exploitation. Rather, it would be because of the pollution of the world’s oceans by heavy industries and industrialised agriculture in urban areas, far from the waters around the Faroes (Fielding 2010).
By-catch
Pilot whales are taken as by-catch in some fisheries on both sides of the Atlantic, including long lines, gillnet, driftnet, purse seines, mid-water and bottom trawls. They were taken in significant numbers in the Mediterranean driftnet fishery for swordfish (Xiphias gladius) in the 1980s, however limitations on net size imposed in 1990 have probably ameliorated this problem (Notarbartolo-di-Sciara 1990, Natale and Notarboartolo-di-Sciara 1994). Currently, by-catch of pilot whales in the eastern Atlantic is likely not of concern. For the period 2008–2019, the ICES Working Group on Bycatch of Protected Species reports only a few by-catches of pilot whales in European Fisheries (all in the Atlantic), in mid-water trawlers from the Faroe Islands, France, Germany and the Netherlands (ICES 2019). As for other marine mammal species, there are, however, likely more whales by-caught than reported.

Solve the bycatch problem: fish wireless. © wulffmorgenthaler
Competition with fisheries

Russian Fishing Vessel. © Monika von Minden, IMR
Pilot whales are one the most abundant cetaceans in the North Atlantic, and their impact on the ecosystem as consumers is likely substantial. In the central North Atlantic around Iceland, pilot whales are the third most important marine mammal predator in terms of total biomass, consuming close to 300,000 tonnes of fish, and 1,100 thousand tonnes of squid per year (Sigurjónsson and Víkingsson 1997). They are also one of the most important marine mammal predators in the western North Atlantic (Gannon et al. 1997a,b). Much of this consumption is concentrated on species presently of little importance to commercial fisheries, so direct competition with fisheries is likely to be minimal.
Conversely, prey important to pilot whales are targeted by industrial fisheries (e.g., squids, mackerel, blue whiting and herring). The availability of these for whales might therefore be reduced by commercial fisheries. Long-finned pilot whales feed on a wide variety of prey and appear to be able to adjust their diet in response to changes in prey abundance (Desportes and Mouritsen 1993, Santos et al. 2013). They are therefore likely to be less vulnerable to prey depletion than more specialised species. However, pilot whale diet includes several high-quality species. The quality of food being dictated by the cost of living for each species means that shifting to food of lower-quality species (species with lower energy densities per mass unit) may negatively affect the population dynamics of a species (Spitz et al. 2011, 2012).
Squid
Fisheries for squid are widespread on both sides of the North Atlantic, targeting species which are the main prey eaten by pilot whales in the area: short-finned squid off Newfoundland, long-finned squid (Loligo pealeii) off the US coast, European flying squid off the Faroe Islands, Norway and Portugal, curled octopus (Eledone cirrhosa) off Spain and Portugal. This raises the possibility of prey depletion for pilot whales.
The abundance of squid available to fisheries is known to exhibit dramatic yearly fluctuations, with no obvious pattern. Periods of either high or low abundance may last for several years. These changes would be determined by the interaction of fluctuating year-class strength and varying hydrographic conditions influencing natural mortality and the distribution of the early life stages (Arnold 1979, Fisheries and Oceans Canada 2013).
Blue whiting
The intensified pelagic fishery targeting blue whiting west of the British Isles is likely to be one of the factors reinforcing the apparent decrease in abundance of pilot whales since the 1990s (Hátun and Gaard 2010). The blue whiting is preyed upon by both the flying squid and the pilot whale.
Acoustic pollution

Danish Fisheries Inspection vessel. © Pernille Tønnesen, HVIDBJØRNEN
This species, like many beaked whales, is likely to be vulnerable to loud anthropogenic sounds, such as those generated by navy sonar and seismic exploration (Cox et al. 2006), and almost all behavioural responses are expected to result in a change in dive pattern (Sivle et al. 2012). Indeed, intense military sonar signals should be audible to cetaceans over large distances given the efficient propagation of sound and the sensitive hearing capabilities of cetaceans.
Exposure to military sonar pulses is associated with changes in behaviour in pilot whales, suggesting acoustic disturbance. Changes in vocalisations, traveling, surfacing and diving/foraging behaviours have been observed, the latter potentially reducing the foraging efficiency of the affected animals (Rendell and Gordon 1999, Miller et al. 2011, 2012, Sivle et al. 2012).
Pilot whales may be less sensitive to sonar exposure, compared to species such as killer whales and beaked whales, but they still respond behaviourally to military sonar (Antunes et al. 2014). Hearing impairment or a behavioural response to underwater military explosions occurring in Scotland have been associated with two mass strandings in Scotland (Brownlow et al. 2015).
Ship traffic and whale watching
The primary natural habitat of the long-finned pilot whale is in deep offshore waters where they are usually less exposed to maritime traffic and unavailable for whale watching. These activities are therefore likely not of concern in the eastern Atlantic but could be in the Mediterranean where some collisions have been recorded (Verborgh et al. 2016).
Maritime traffic could affect the main prey of pilot whales. Cephalopods respond to low frequency sounds, such as the ones emitted by large container ships, by altering their breathing rate and moving away from loud areas. Permanent acoustic trauma can be inflicted (André et al. 2011).
Oceanographic changes
The impact of global climate change on the marine environment may affect long-finned pilot whales, and induce changes in the species’ range, abundance and/or migration patterns (Learmont et al. 2006, Fielding 2010). In particular, pilot whales may be affected indirectly, through their prey, as the migration and survival of squid is very temperature-dependent (Lacoue-Labarthe et al. 2016).
MacLeod et al. (2005) suggest that the warming of local waters in the late 1980s has, indeed, led to changes in the cetacean community of northwest Scotland, with a decline in occurrence of cold water species, including pilot whales, an increase in the occurrence of existing warm water species and the addition of new warm water species to the community. They predicted that if the temperature rise continued, colder water species like the pilot whale might be entirely lost from the northwest Scottish cetacean community.
A clear link has been identified between the abundance of long-finned pilot whales and the marine climate in the northeastern Atlantic throughout the last three centuries (Hoydal and Lastein 1993, Hátun et al. 2009). During warm periods, the whales are observed in high abundance while they can be completely absent from the region during cold periods. The linkage between the marine climate and the abundance of pilot whales probably involves their main prey items, the flying squid and the large but highly variable blue whiting stock (Hátun and Gaard 2010). This link, however, was broken after the 1980s, when warming and an increase in the blue whiting stock were not followed by an increase in the abundance of flying squids and pilot whales. Hátun and Gaard (2010) identified potential causes rooted in global warming and an intensified pelagic fishery, which may collectively explain this apparent disruption.
Research in NAMMCO Member Countries
All NAMMCO member countries, as well as Canada, have participated in the NASS and T-NASS surveys, and the pilot whale has been one of the target species (see under Current Abundance and Trends). These surveys are coordinated through the Scientific Committee of NAMMCO. In addition to this work on abundance and trends, the Faroe Islands also conducts research on ecology, population structure, movements, the accumulation of contaminants and the impacts of climate change on pilot whales.
Faroe Islands
An intensive international research programme on the ecology and status of the long-finned pilot whale off the Faroe Islands and the impact of hunting on the population was initiated and supported by the Faroese Government in 1986, under the auspices of the IWC and UNEP (Desportes 1988, 1990, Desportes et al. 1992b). Some of the results were compiled in the first monography on Northern Hemisphere pilot whales (Donovan et al. 1993).
On-going biological sampling of pilot whales landed in the Faroe Islands, together with satellite tracking projects and regular sighting surveys, are important tools that provide updated information for management to ensure that the pilot whale catch remains sustainable.
Following pilot whale movements through satellite trackingSatellite transmitters have been successfully attached to pilot whales from 10 different schools since July 2000 (see table to the right). In 2020, animals from three pilot whale groups were tagged, with two groups tracked simultaneously, for the first time (Mikkelsen pers. comm.). The plan is now to analyse the data that has been achieved so far, before initiating any new tagging studies (National Progress Report Faroe Islands 2020). In each case, the schools of whales were driven into a bay, the tags attached to the selected whales, and the entire school driven back out to sea again. The maps below show the overall movements of the whales from some of the tracking events. These tagging experiments are very important because they shed light on the extent of the population affected by the Faroese catch. As described under the section on stock status the effects of the catch are very different if the catch comes from a population restricted to the Faroe Islands area or from a population spread over a wider geographical area. |
Satellite tagging of long-finned pilot whales off the Faroe Islands
| Date & location | Pod size | No. of tagged whales | Max. period of contact (days) |
|---|---|---|---|
| 15 July 2000, Sandavágur (West) | 80 | 4 | 47 |
| 25 August 2004, Sandavágur (West) | 80 | 7 | 133 |
| 25 May 2011, Hvannasund (North) | 40 | 8 | 14 |
| 2 October 2012, Vágur (South) | 45 | 6 | 125 |
| 24 August 2015, Fuglafjørður | 7 | 5 | 112 |
| 5 September 2018, Bø, Vágoy | 15 | 4 | 50 |
| 7 July 2019, Gøtu | 16 | 5 | 178 |
| 6 June 2020, Bøur | 30 | 2 | 225 |
| 16 June 2020, Tórshavn | 12 | 4 | 75 |
| 13 November 2020, Vestmanna | 10 | 3 | (to be confirmed) |
Satellite-monitored movements of long-finned pilot whale
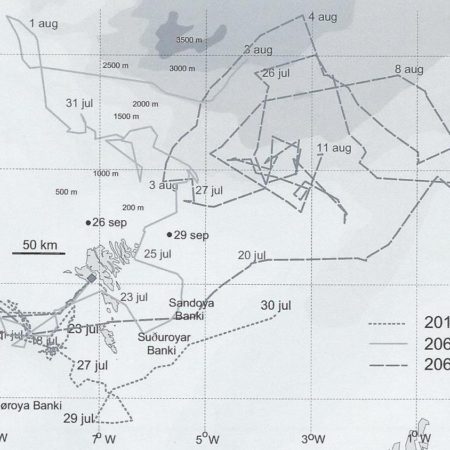
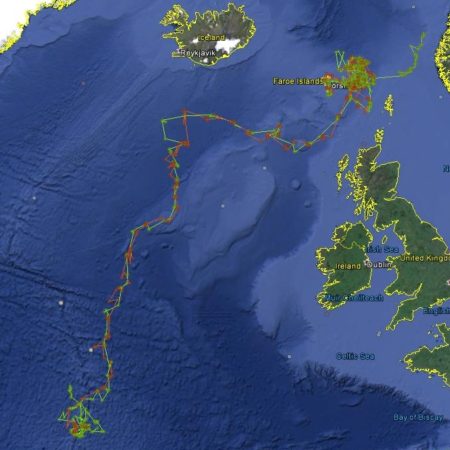
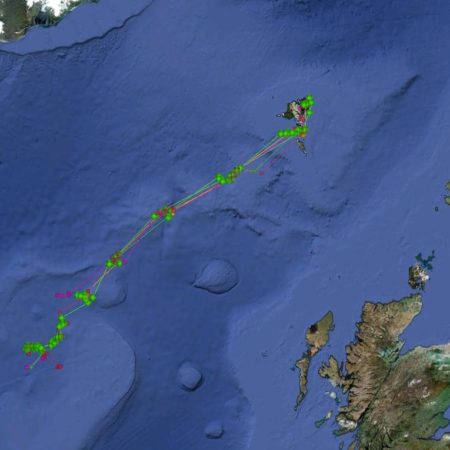
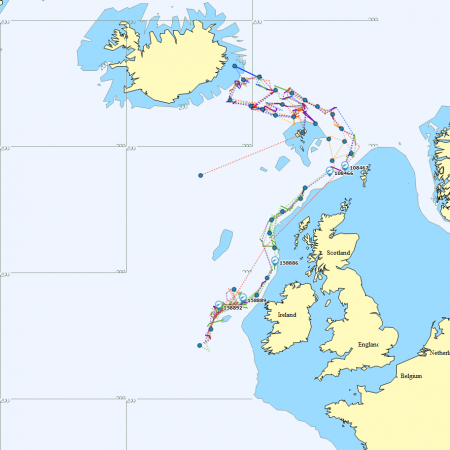
Ongoing sampling of pilot whale catches
The measurement of skin values, which has been traditionally used in Faroese whaling (see under Hunting and Utilisation), continue to be recorded. This measure, together with the sex and total body length, which have been recorded for most pilot whales caught since 1984, allows researchers to follow trends in the size of the whales landed.
The Faroese Museum of Natural History also conducts a small-scale opportunistic sampling of the catch to monitor trends in life history parameters and feeding habits. In 2019, the Natural History Museum of the Faroe Islands collected biological samples from a total of 154 pilot whales (collected during 4 different grinds) to be analysed for age and reproduction parameters (National Progress Report Faroe Islands 2019). In addition, stomachs and tissues from some of the same animals were stored for diet, genetic and contaminant studies. The Environment Agency also took muscle, blubber, liver and kidney tissue samples from 81 individuals during the same hunts. In addition, muscle, blubber, liver and kidney samples were taken from 6 foetuses from the grind in Sandavágur. The Environment Agency regularly collects pilot whale samples for a tissue bank, with an aim to take samples from three schools a year, with around 25 individuals from each. Overall, significantly fewer samples were taken in 2020, because of fewer pilot whale hunts due to COVID-19 (National Progress Report Faroe Islands 2020)
Level of contaminants in pilot whales
Samples of pilot whale tissue are regularly taken by the Faroese Environment Agency to examine and follow the levels of heavy metals in the meat and organochlorines in the blubber of the whales. In recent years, samples for RNA and DNA analyses have been included in this program.
The focus of the monitoring of muscle and blubber is to elucidate possible changes in concentrations over time and therefore exposure of the Faroese human population utilising pilot whale blubber and meat for food. The focus of the monitoring of heavy metals in kidney and liver tissues is to follow the possible risk to the pilot whales of elevated concentrations, which are known to impair reproduction in many species. The monitoring of pollutants in pilot whales is focused on juvenile males, so as to minimise variability that stems from sex/age related biological processes.
Since 2008, the monitoring data collected by the Environment Agency has been communicated to AMAP and are available online, under the heading ). ENVOFAR is a cooperation of Faroese institutions that works actively to describe and study the environment in the AMAP and CAFF (Conservation of Arctic Flora and Fauna) working groups under the Arctic Council. ENVOFAR). ENVOFAR is a cooperation of Faroese institutions that works actively to describe and study the environment in the AMAP and CAFF working groups under the Arctic Council.
In 2019, samples of pilot whales collected in the Faroe Islands were also included in a Nordic Council of Ministers supported study of UV-filters, as arranged and coordinated by the Nordic Screening Group.
Recent research on contaminants using pilot whale samples from the Faroe Islands has indicated that partitioning to phospholipids is an important mechanism of bioaccumulation for long-chain poly- and perfluoroalkyl substances in marine mammals (Dassuncao et al. 2019). Furthermore, the composition of mercury isotopes in the liver of adult whales is different from younger whales and that reduced availability of selenium may drive mercury redistribution among pilot whale tissues (Li et al. 2020).



Iceland
In 2019 work began to compile the first photo-identification catalogue of pilot whales in Iceland. Studies on the ecology of pilot whales have also started by collecting samples of all available stranded animals in the MFRI tissue bank to analyse for stable isotopes of carbon and nitrogen. The aim of this work is to gather knowledge to help understand the factors driving the unusually high number of strandings that occurred in 2019, as well as the occurrence of the species in Icelandic coastal waters, the prey it targets and whether this changes over times (National Progress Report Iceland 2019). In addition to this, the behaviour of pilot whales during interactions with killer whales in Vestmannaeyjar was studied in 2020 (National Progress Report Iceland 2020).
Abend, A.G. and Smith, T.D. (1997). Differences in ratios of stable isotope ratios of carbon and nitrogen between long-finned pilot whales (Globicephala melas), and their primary prey in the western North Atlantic. ICES Journal of Marine Science, 54(3), 500–503. http://dx.doi.org/10.1006/jmsc.1996.0192
Aguilar, A., Jover, L. and Borrell, A. (1993). Hetergeneities in organochlorine profiles of Faroese longfinned pilot whales: indication of segregation between pods? Report of the International Whaling Commission, sp 14, 359–368.
Aguilar de Soto, N., Johnson, M.P., Madsen, P.T., et al. (2008). Cheetahs of the deep sea: deep foraging sprints in short-finned pilot whales off Tenerife (Canary Islands). Journal of Animal Ecology, 77, 936–947. http://dx.doi.org/10.1111/j.1365-2656.2008.01393.x
Amos, B., Barrett, J. and Dover, G.A. (1991). Breeding behaviour of pilot whales revealed by DNA fingerprinting. Heredity, 67(1), 49–55. https://doi.org/10.1038/hdy.1991.64
Amos, B., Bloch, D., Desportes, G., et al. (1993a). A review of molecular evidence relating to social organisation and breeding system in the long-finned pilot whale. Report of the International Whaling Commission, sp 14, 209–217.
Amos, B., Schlotterer, C. and Tautz, D. (1993b). Social structure of pilot whales revealed by analytical DNA profiling. Science, 260, 670–672. http://dx.doi.org/10.1126/science.8480176
Andersen, L.W. (1990). The population structure and sex determination of the long-finned pilot whale, Globicephala melas, and the harbour porpoise, Phocoena phocoena. Ph.D. thesis. Aarhus University, Denmark. 50pp.
Andersen, L.W. (1993). Further studies on the population structure of the long-finned pilot whale, Globicephala melas, of the Faroe Islands. Report of the International Whaling Commission, sp 14, 219–231.
Andersen, L.W. and Siegismund, H.R. (1994). Genetic evidence for migration of males between schools of the long-finned pilot whale, Globicephala melas, of the Faroe Islands. Marine Ecology Progress Series, 105, 1–7. https://doi.org/10.3354/meps105001
Anonymous. (2013). Whaling in the Faroe Islands. Available at https://www.whaling.fo/
André, M., Solé, M., Lenoir, M. et al. (2011) Low-frequency sounds induce acoustic trauma in cephalopods. Frontiers in Ecology and the Environment, 9, 489–493. https://doi.org/10.1890/100124
Andreasen, B., Hoydal, K., Mortensen, R., et al. (2019). AMAP Faroe Islands 2013–2016: Heavy Metals and POPs Core Programme. Umhvørvisstovan, Argir, Faroe Islands, 103 pp.
Antunes, R., Kvadsheim, P.H., Lam, F.P.A., et al. (2014). High thresholds for avoidance of sonar by free-ranging long-finned pilot whales (Globicephala melas). Marine Pollution Bulletin, 83, 165–180. http://dx.doi.org/10.1016/j.marpolbul.2014.03.056
Aoki, K., Sakai, M., Miller, P.J.O, et al. (2013). Body contact and synchronous diving in long- finned pilot whales. Behavioural Processes, 99, 12–20. http://dx.doi.org/10.1016/j.beproc.2013.06.002
Arctic Monitoring and Assessment Programme (AMAP). (2004). AMAP assessment 2002 – Persistent organic pollutants in the Arctic. Oslo, Norway. 310pp. Available at http://www.amap.no
Arctic Monitoring and Assessment Programme (AMAP). (2005). AMAP assessment 2002: Heavy metals in the Arctic. Oslo, Norway. 265 pp. Available at http://www.amap.no
Arctic Monitoring and Assessment Programme (AMAP). (2018a). AMAP Assessment 2018: Biological Effects of Contaminants on Arctic Wildlife and Fish. Summary for Policy-Makers. https://www.amap.no/documents/download/3297/inline
Arctic Monitoring and Assessment Programme (AMAP). (2018b). AMAP Assessment 2018: Biological Effects of Contaminants on Arctic Wildlife and Fish. Oslo, Norway. vii+84pp
Arnold, G.P. (1979). Squid – A review of their biology and fisheries. Laboratory leaflet nr. 48. Lowestoft. 37pp.
Balbuena, J.A., Aznar, F.J., Fernández, M. and Raga, J.A. (1995). Parasites as indicators of social structure and stock identity of marine mammals. Developments in Marine Biology, 4, 133–139. https://doi.org/10.1016/S0163-6995(06)80017-X
Balbuena, J.A. and Raga J.A. (1991). Ecology and host relationships of the whale-louse Isocyamus delphini (Amphipoda: Cyamidae) parasitizing long-finned pilot whales (Globicephala melas) off the Faroe Islands (Northeast Atlantic). Canadian Journal of Zoology, 69, 141–145. https://doi.org/10.1139/z91-021
Balbuena, J.A. and Raga, J.A. (1993). Intestinal helminth communities of the long-finned pilot whale (Globicephala melas) off the Faroe Islands. Parasitology, 106, 327–333. http://dx.doi.org/10.1017/S0031182000075156
Balbuena, J.A. and Raga, J.A. (1994). Intestinal helminths as indicators of segregation and social structure of pods of long-finned pilot whales (Globicephala melas) off the Faroe Islands. Canadian Journal of Zoology, 72, 443–448. http://dx.doi.org/10.1139/z03-127
Baird, R.W., Borsani, J.F., Hanson, M.B. and Tyack, P. (2002). Diving and night-time behaviour of long- finned pilot whales in the Ligurian Sea. Marine Ecology Progress Series, 237, 301–305. https://doi.org/10.3354/meps237301
Bjurlid, F., Dam, M., Hoydal, K. and Hagberg, J. (2018). Occurrence of polybrominated dibenzo-p-dioxins, dibenzofurans (PBDD/Fs) and polybrominated diphenyl ethers (PBDEs) in pilot whales (Globicephala melas) caught around the Faroe Islands. Chemosphere, 195, 11–20. https://doi.org/10.1016/j.chemosphere.2017.12.044
Bloch, D. (1994). Pilot whales in the North Atlantic. Age, growth and social structure in Faroese grinds of the long-finned pilot whale, Globicephala melas. Doctoral dissertation, Lund University, Sweden.
Bloch, D. (1998). A review of marine mammals observed caught or stranded over the last two centuries in Faroese Waters. Shetland Sea Mammal Report, 1997, 15–37.
Bloch, D. (2007). Grindehvalen og Færøernes grindefangst. Føroya Náttúrugripasavn, Tórshavn Færøerne. 52pp.
Bloch, D., Desportes, G., Hoydal, K. and Jean, P. (1990). Pilot whaling in the Faroe Islands: July 1986–July 1988. North Atlantic Studies, 2, 36–44.
Bloch, D., Desportes, G., Mouritsen, R. et al. (1993a). An introduction to studies on the ecology and status of the long-finned pilot whale (Globicephala melas) off the Faroe Islands, 1986–1988. Report of the International Whaling Commission, sp 14, 1–32.
Bloch, D., Heide-Jørgensen, M.P., Stefansson, E. et al. (2003). Short-term movements of long-finned pilot whales Globicephala melas around the Faroe Islands. Wildlife Biology, 9, 47–58. https://doi.org/10.2981/wlb.2003.007
Bloch, D. and Lastein, L. (1993). Morphometric segregation and long-finned pilot whale in the eastern and western North Atlantic. Ophelia, 38, 55–68. http://dx.doi.org/10.1080/00785326.1993.10429924
Bloch, D., Lockyer, C. and Zachariassen, M. (1993b). Age and growth parameters of the long-finned pilot whale off the Faroe Islands. Report of the International Whaling Commission, sp 14, 163–208.
Bloch, D., Zachariassen, M. and Zachariassen, P. (1993c). Some external characters of the long-finned pilot whale off the Faroe Islands and a comparison with the short-finned pilot whale. Report of the International Whaling Commission, sp 14, 117–135.
Borrel, A. (1993). PCB and DDTs in blubber of cetaceans from the northeastern North Atlantic. Marine Pollution Bulletin, 16, 146–151. http://dx.doi.org/10.1016/0025-326X(93)90125-4
Borrel, A. and Aguilar, A. (1993). DDT and PCB pollution in blubber and muscle of long-finned pilot whales from the Faroe Islands. Report of the International Whaling Commission, sp 14, 351–358.
Borrell, A., Bloch, D. and Desportes, G. (1995). Age trends and reproductive transfer of organochlorine compounds in long-finned pilot whales from the Faroe Islands. Environmental Pollution, 88, 283–292. http://dx.doi.org/10.1016/0269-7491(95)93441-2
Brownlow, A., Baily, J., Dagleish, M. et al. (2015) Investigation into the long-finned pilot whale mass stranding event, Kyle of Durness, 22nd July 2011. Report to Defra and Marine Scotland, Inverness.
Buckland, S.T., Bloch, D., Cattanach, K.L. et al. (1993). Distribution and abundance of long-finned pilot whales in the North Atlantic, estimated from NASS-1987 and NASS-89 data. Report of the International Whaling Commission, sp 14, 33–50.
Bustamante, P., Caurant, F., Fowler, S.W. and Miramand, P. (1998). Cephalopods as a vector for the transfer of cadmium to top marine predators in the north-east Atlantic Ocean. Science of the Total Environment, 220, 71–80. http://dx.doi.org/10.1016/S0048-9697(98)00250-2
Canadas, A. and Sagarminaga, R. (2000). The northeastern Alboran Sea, an important breeding and feeding ground for long-finned pilot whale (Globicephala melas) in the Mediterranean Sea. Marine Mammal Science, 16(3), 513–529. http://dx.doi.org/10.1111/j.1748-7692.2000.tb00948.x
Caurant, F., Amiard-Triquet, C. and Amiard J.-C. (1993). Factors influencing the accumulation of metals in pilot whales (Globicephala melas) off the Faroe Islands. Report of the International Whaling Commission, sp 14, 369–390.
Caurant, F. and Amiard-Triquet, C. (1995). Cadmium contamination in pilot whales Globicephala melas: source and potential hazard to the species. Marine Pollution Bulletin, 30, 207–210. http://dx.doi.org/10.1016/0025- 326X(94)00126-T
Caurant, F., Amiard J.C., Amiard-Triquet, C. and Sauriau, P.G. (1994). Ecological and biological factors controlling the concentrations of trace elements (As, Cd, Cu, Hg, Se, Zn) in delphinids Globicephala melas from the North Atlantic Ocean. Marine Ecology Progress Series, 103, 207–219. https://doi.org/10.3354/meps103207
Caurant, F., Navarro, M. and Amiard, J.C. (1996). Mercury in pilot whales: possible limits to the detoxification process. Science of the Total Environment, 186, 95–104. http://dx.doi.org/10.1016/0048- 9697(96)05087-5
Choi, A.L., Weihe, P., Budtz-Jørgensen, E. et al. (2009). Methylmercury exposure and adverse cardiovascular effects in Faroese whaling men. Environmental Health Perspectives, 117, 367–372. http://dx.doi.org///10.1289/ehp.11608
Cox, T.M., Ragen, T.J., Read, A.J. et al. (2006). Understanding the impacts of anthropogenic sound on beaked whales. Journal of Cetacean Research and Management, 7(3), 177–187. https://archive.iwc.int/pages/search.php?search=%21collection15&k=
Curé, C., Antunes, R., Samarra, F. et al. (2012). Pilot whales attracted to killer whale sounds: acoustically-mediated interspecific interactions in cetaceans. PLoS ONE, 7(12), e52201. http://dx.doi.org/10.1371/journal.pone.0052201
Curé C, Isojunno S, Vester HI et al. (2019) Evidence for discrimination between feeding sounds of familiar fish and unfamiliar mammal-eating killer whale ecotypes by long-finned pilot whales. Animal Cognition, 22, 863–882. https://doi.org/10.1007/s10071-019-01282-1
Culik, B.M. (2011). Odontocetes – The toothed whales. CMS Technical Series No. 24. UNEP/CMS/ASCOBANS, Bonn, Germany. 311pp. https://www.ascobans.org/en/publication/odontocetes-toothed-whales
Dam, M. and D. Bloch. (2000). Screening of mercury and persistent organochlorine pollutants in long- finned pilot whale (Globicephala melas) in the Faroe Islands. Marine Pollution Bulletin, 40, 1090–1099. http://dx.doi.org/10.1016/S0025-326X(00)00060-6
Dassuncao, C., Pickard, H., Pfohl, M. et al. (2019). Phospholipid levels predict the tissue distribution of poly-and perfluoroalkyl substances in a marine mammal. Environmental Science and Technology Letters, 6(3), 119–125. https://doi.org/10.1021/acs.estlett.9b00031
Desportes, G. (1988). Ecology and status of pilot whales off the Faroe Islands. ECS Newsletter, 3, 2–4.
Desportes, G. (1985). La nutrition des odontocètes en Atlantique Nord-Est (côtes françaises et iles Féroé). PhD thesis. Université de Poitiers, France. 190pp.
Desportes, G. (1990). Pilot whale research in the Faroe Islands: presentation and preliminary results. North Atlantic Studies, 2(1+2), 47–54.
Desportes, G., Andersen, L.W., Aspholm, P.E. et al. (1992a). A note about a male- only pilot whale school observed in Faroe Islands. Fróðskaparrit, 40, 27–33.
Desportes, G., Andersen, L.W. and Bloch, D. (1994a). Variation in foetal and postnatal sex ratios in long- finned pilot whales. Ophelia, 39(3), 183–196. http://dx.doi.org/10.1080/00785326.1994.10429543
Desportes, G., Bloch, D., Andersen, L.W. and Mouritsen, R. (1992b). The international research programme on the ecology and status of the long-finned pilot whale off the Faroe Islands: Presentation, results and reference. Fróðskaparrit, 40, 9–29.
Desportes, G. and Mouritsen, R. (1993). Preliminary results on the diet of long-finned pilot whales off the Faroe Islands. Report of the International Whaling Commission, sp 14, 305–324.
Desportes, G., Saboureau, M. and Lacroix, A. (1994b). Growth-related changes in testicular mass and plasma testosterone concentrations in long-finned pilot whales, Globicephala melas. Journal of Reproduction and Fertility, 102, 237–244. http://dx.doi.org/10.1530/jrf.0.1020237
Desportes, G., Saboureau, M. and Lacroix, A. (1993). Reproductive maturity and seasonality of male long- finned pilot whales, off the Faroe Islands. Report of the International Whaling Commission, sp 14, 233–262.
de Stephanis, R., Verborgh, P., Pérez, S. et al (2008) Long-term social structure of long-finned pilot whales (Globicephala melas) in the Strait of Gibraltar. Acta Ethologica, 11, 81–94. https://doi.org/10.1007/s10211-008-0045-2
de Stephanis, R., Giménez, J., Esteban, R. et al (2015) Mobbing-like behavior by pilot whales towards killer whales: a response to resource competition or perceived predation risk? Acta Ethologica, 18, 69–78. https://doi.org/10.1007/s10211-014-0189-1
Donovan, G.P., Lockyer, C. and Martin, A.R. (eds). (1993). Biology of Northern Hemisphere pilot whales. Report of the International Whaling Commission, sp 14, 479pp.
Eskesen, I., Wahlberg, M., Simon, M. and Larsen, O.N. (2011). Comparison of echolocation clicks from geographically sympatric killer whales and long-finned pilot whales (L). Journal of the Acoustical Society of America, 130(1), 9-12. http://dx.doi.org/10.1121/1.3583499
Evans, P.G.H. (1980). Cetaceans in British waters. Mammal Review, 10, 1–52. http://dx.doi.org/10.1111/j.1365-2907.1980.tb00232.x
Fielding, R. (2010). Environmental change as a threat to the pilot whale hunt in the Faroe Islands. Polar Research, 29, 430–438. http://dx.doi.org/10.1111/j.1751-8369.2010.00168.x
Fisheries and Ocean Canada. (2013). Underwater World – Squid. Modified 22-04-2013. http://www.dfo- mpo.gc.ca/science/publications/uww-msm/articles/squid-calmar-eng.html
Foote, A.D. (2008). Mortality rate acceleration and post-reproductive lifespan in matrilineal whale species. Biology Letters, 4(2), 189–191. http://dx.doi.org/10.1098/rsbl.2008.0006
Fullard, K.J., Early, G., Heide-Jørgensen, M.P. et al. (2000). Population structure of long-finned pilot whales in the North Atlantic: a correlation with sea surface temperature? Molecular Ecology, 9, 949–958. http://dx.doi.org/10.1046/j.1365-294x.2000.00957.x
Gannon, D.P., Read, A.J., Craddock, J.E. et al. (1997a). Feeding ecology of the long-finned pilot whale in the western North Atlantic. Marine Ecology Progress Series, 148, 1-10. http://dx.doi.org/10.3354/meps148001
Gannon, D.P., Read, A.J., Craddock, J.E. and Mead, J.G. (1997b). Stomach contents of long-finned pilot whales (Globicephala mela) stranded on the US mid-Atlantic coast. Marine Mammal Science, 13, 405-413. http://dx.doi.org/10.1111/j.1748-7692.1997.tb00648.x
Gauffier, P., Verborgh, P. & Desportes, G. 2023. Globicephala melas (Europe assessment). The IUCN Red List of Threatened Species 2023: e.T9250A213293843. Accessed on 22 December 2023.
Giorli, G, Au, W.W.L. and Neuheimer, A. (2016). Differences in foraging activity of deep sea diving odontocetes in the Ligurian Sea as determined by passive acoustic recorders. Deep Sea Research Part I: Oceanographic Research Papers, 107, 1–8. https://doi.org/10.1016/j.dsr.2015.10.002
Grandjean, P., Henriksen, J.E., Choi, A.L. et al. (2011). Marine food pollutants as a risk factor for hypoinsulinemia and type 2 diabetes. Epidemiology, 22, 410–417. http://dx.doi.org/10.1097%2FEDE.0b013e318212fab9
Hammond, P., Lacey, C., Gilles, A. et al. (2017). Estimates of cetacean abundance in European Atlantic waters in summer 2016 from the SCANS-III aerial and shipboard surveys. Wageningen University, 2017. 40 p.
https://www.wur.nl/en/Publication-details.htm?publicationId=publication-way-353230323937
Hammond, P.S., Macleod, K., Gillespie, D. et al. (2009). Cetacean Offshore Distribution and Abundance in the European Atlantic (CODA).
Anon_2009_CODA_Final_Report.pdf
Hansen, R. G. and Heide-Jørgensen, M.P. (2013). Spatial trends in abundance of long-finned pilot whales, white beaked dolphins and harbour porpoises in West Greenland. Marine Biology, 160(11), 2929-2941. http://dx.doi.org/10.1007/s00227-013-2283-8
Hansen, R.G., Boye, T.K., Larsen, R.S. et al. (2018). Abundance of whales in West and East Greenland in summer 2015. NAMMCO Scientific Publications, 11. https://doi.org/10.7557/3.4689
Hátún, H. and Gaard, E. (2010). Marine climate, squid and pilot whales in the northeastern Atlantic. In S.-A. Bengston, P. Buckland, P.H. Enckell and A.M. Fosaa (eds.), Dorete – her book, 50–68. Tórshavn: Faroe University Press.
Hátún, H., Payne, M.R., Beaugrand, G. et al. (2009). Large bio-geographical shifts in the north-eastern Atlantic Ocean. From the subpolar gyre, to blue whiting and pilot whales. Prog. Oceanography, 80, 149–162. http://dx.doi.org/10.1016/j.pocean.2009.03.001
Hay, K. (1982). Aerial line-transect estimates of abundance of humpback, fin, and long-finned pilot whales in the Newfoundland-Labrador area. Report of the International Whaling Commission, 32, 475–486.
Hayes, S.A., Josephson, E., Maze-Foley, K. and Rosel, P.E. (2020). US Atlantic and Gulf of Mexico Marine Mammal Stock Assessments – 2019. NOAA Technical Memorandum NMFS-NE-264. 479pp. https://www.fisheries.noaa.gov/national/marine-mammal-protection/marine-mammal-stock-assessment-reports-region
Hoydal, K. and Lastein, L. (1993). Analysis of Faroese catches of pilot whales (1709-1992), in relation to environmental variations. Report of the International Whaling Commission, sp 14, 89-106.
Hoydal, K.S., Letcher, R.J., Blair, D.A.D. et al. (2015). Legacy and emerging organic pollutants in liver and plasma of long-finned pilot whales (Globicephala melas) from waters surrounding the Faroe Islands. Science of The Total Environment, 520, 270–285. https://doi.org/10.1016/j.scitotenv.2015.03.056
International Council for the Exploration of the Sea (ICES). (1993). Report of the study group on long- finned pilot whales, Copenhagen, Denmark, 30 August – 3 September. ICES (International Council for the Exploration of the Sea) CM 1993/N:5.
International Council for the Exploration of the Sea (ICES). (1996). Report of the study group on longfinned pilot whales. Cambridge, United Kingdom. 22-26 April. ICES CM 1996/A:6.
Jákupsstovu, S.H.Í. (2002). The pelagic fish stocks, pilot whales and squid in Faroese waters – migration pattern, availability to fisheries and possible links to oceanographic events. ICES CM 2002/N:07, 37pp.
Jefferson, T.A., Leatherwood, S. and Webber, M.A. (1993). FAO species identification guide. Marine mammals of the World. UNEP/FAO, Rome. 320pp.
Joensen, J.P. (1976). Pilot whaling in the Faroe Islands. Ethnologica Scandinavia. 43pp.
Joensen, J.P. (2009). Pilot whaling in the Faroe Islands: History, ethnography, symbol. Tórshavn, Faroe University Press.
Kerins, S. (2008). Whaling in the Faroes. Ph.D. thesis, Griffith School of Environment, Griffith University, QLD Australia. Available at https://trove.nla.gov.au/work/163050846?q&versionId=177692067
Kok, A. (2012). The importance of vocal communication in long-finned pilot whales. http://www.fonaconservation.nl/index.php/en/projects-completed/16-projecten-2012/61-het- belang-van-vocale-communicatie-voor-de-gewone-griend
Lacoue-Labarthe, T., Nunes, P.A.L.D., Ziveri, P. et al. (2016). Impacts of ocean acidification in a warming Mediterranean Sea: An overview. Regional Studies in Marine Science, 5,1–11. https://doi.org/10.1016/j.rsma.2015.12.005
Laran, S., Authier, M., Blanck, A. et al. (2017). Seasonal distribution and abundance of cetaceans within French waters- Part II: The Bay of Biscay and the English Channel. Deep Sea Research Part II: Topical Studies in Oceanography, 141, 31–40. https://doi.org/10.1016/j.dsr2.2016.12.012
Lauriano, G., Di Guardo, G., Marsili, L., et al (2014) Biological threats and environmental pollutants, a lethal mixture for mediterranean cetaceans? Journal of the Marine Biological Association of the UK, 94, 1221–1225. https://doi.org/10.1017/S0025315413000714
Lawson, J.W. and Gosselin, J.-F. (2018). Estimates of cetacean abundance from the 2016 NAISS aerial surveys of eastern Canadian waters, with a comparison to estimates from the 2007 TNASS. SC/25/AE/09 for the NAMMCO Scientific Committee.
Lawson, J.W. and Gosselin, J.-F. (2009). Distribution and preliminary abundance estimates for cetaceans seen during Canada’s Marine Megafauna Survey – A component of the 2007 TNASS. Canadian Science Advisory Secretariat. Res. Doc. 2009/031. 28 pp. http://www.dfo-mpo.gc.ca/csas-sccs/publications/resdocs-docrech/2009/2009_031-eng.htm
Lesson, R.P. (1828) Histoire naturelle générale et particulière des mammifères et des oiseaux découverts depuis 1788 jusqu’à nos jours. Cétacés. Jules Didot ainé, Paris
Li, M., Juang, C.A., Ewald, J.D. et al. (2020). Selenium and stable mercury isotopes provide new insights into mercury toxicokinetics in pilot whales. Science of the Total Environment, 710, 136325. https://doi.org/10.1016/j.scitotenv.2019.136325
MacLeod, C.D., Bannon, S.M., Pierce, G.J. et al. (2005). Climate change and the cetacean community of north-west Scotland. Biological Conservation, 124, 477–483. http://dx.doi.org/10.1016/j.biocon.2005.02.004
Marina, T.I., Marchesi, M.C. and Goodall, R.N.P. (2019). Long‐finned pilot whale (Globicephala melas, Traill 1809) subspecies in the Atlantic Ocean: Are there differences in their skulls? Marine Mammal Science, 35, 660–676. https://doi.org/10.1111/mms.12548
Martin, A.R., Reynolds, P. and Richardson, M.G. (1987). Aspects of the biology of pilot whales (Globicephala melaena) in recent mass strandings on the British coast. Journal of Zoology, 211, 11–23. http://dx.doi.org/10.1111/j.1469-7998.1987.tb07449.x
Martin, A.R. and Rothery, P. (1993). Reproductive parameters of female long-finned pilot whales (Globicephala melas) around the Faroe Islands. Report of the International Whaling Commission, sp 14, 263–304.
Mate, B. (1989). Watching (whale) habits and habitats from earth satellites. Oceanus, 32, 14–18.
Mate, B.R., Lagerquist, B.A., Winsor, M. et al. (2005). Movements and dive habitats of a satellite-monitored longfinned pilot whale (Globicephala melas) in the Northwest Atlantic. Marine Mammal Science, 21, 136–144. https://doi.org/10.1111/j.1748-7692.2005.tb01213.x
Méndez-Fernandez, P., Pierce, G.J., Bustamante, P. et al. (2012). Foraging ecology of five toothed whale species in the Northwest Iberian Peninsula, inferred using carbon and nitrogen isotope ratios. Journal of Experimental Marine Biology and Ecology, 413, 150–158. http://dx.doi.org/10.1016/j.jembe.2011.12.007
Méndez-Fernandez, P., Pierce, G.J., Bustamante, P. et al. (2013). Ecological niche segregation among five toothed whale species off the NW Iberian Peninsula using ecological tracers as multi-approach. Marine Biology, 160(11), 2825–2840. http://dx.doi.org/10.1007/s00227-013-2274-9
Méndez-Fernandez, P., Webster, L., Chouvelon, T., Bustamante, P., Ferreira, M., González, A.F., … and Caurant, F. (2014a). An assessment of contaminant concentrations in toothed whale species of the NW Iberian Peninsula: Part II. Trace element concentrations. Science of the Total Environment, 484(15), 206–217. http://dx.doi.org/10.1016/j.scitotenv.2014.03.001
Méndez-Fernandez, P., Webster, L., Chouvelon, T. et al. (2014b). An assessment of contaminant concentrations in toothed whale species of the NW Iberian Peninsula: Part I. Persistent organic pollutants. Science of the Total Environment, 484(15), 196–205. http://dx.doi.org/10.1016/j.scitotenv.2014.02.045
Mercer, M.C. (1975). Modified Leslie-Delury population models of the long-finned pilot whale (Globicephala melas) and annual production of short-finned squid (Illex illecebrosus) based upon their interaction in Newfoundland. Journal of the Fisheries Research Board of Canada, 32(7), 1145–1154. https://doi.org/10.1139/f75-135
Mikkelsen, B. (2008). Movements and diving of pilot whales in autumn and early winter. NAMMCO SC/15/PW/07. 9pp.
Miller, P.J.O., Antunes, R., Alves, A.C. et al. (2011). The 3S experiments: studying the behavioral effects of sonar on killer whales (Orcinus orca), sperm whales (Physeter macrocephalus), and long-finned pilot whales (Globicephala melas) in Norwegian waters. Scottish Ocean Inst.Tech.Rep. 2011-001. 289pp. http://soi.st-andrews.ac.uk/documents/424.pdf
Miller, P.J.O., Kvadsheim, P.H., Lam, F.P.A. et al. (2012). The severity of behavioral changes observed during experimental exposures of killer (Orcinus orca), long-finned pilot (Globicephala melas), and sperm (Physeter macrocephalus) whales to Naval Sonar. Aquatic Mammals, 38, 362–401. http://dx.doi.org/10.1578/AM.38.4.2012.362
Minton, G., Reeves, R. and Braulik, G. (2018). Globicephala melas. The IUCN Red List of Threatened Species 2018: e.T9250A50356171. https://dx.doi.org/10.2305/IUCN.UK.2018-2.RLTS.T9250A50356171.en
Mitchell, E.D. (1975a). Porpoise, dolphin and small whale fisheries of the world: status and problems. IUCN and Natural Resources, 3, 1–129.
Mitchell, E.D. (1975b). Report of the meeting on smaller cetaceans. Montreal, 1-11 April 1974. Journal of the Fisheries Research Board of Canada, 32(7), 889–983. https://doi.org/10.1139/f75-117
Monteiro, S., Ferreira, M., Vingada, J.V. et al. (2015a) Application of stable isotopes to assess the feeding ecology of long-finned pilot whale (Globicephala melas) in the Northeast Atlantic Ocean. Journal of Experimental Marine Biology and Ecology, 465,56–63. https://doi.org/10.1016/j.jembe.2015.01.007
Monteiro, S.S., Méndez-Fernandez, P., Piertney, S. et al. (2015b) Long-finned pilot whale population diversity and structure in Atlantic waters assessed through biogeochemical and genetic markers. Marine Ecology Progress Series, 536, 243–257. https://doi.org/10.3354/meps11455
Nawojchik, R., Aubin, D.J. St. and Johnson, A. (2003). Movements and dive behavior of two stranded, rehabilitated long-finned pilot whales (Globicephala melas) in the northwest Atlantic. Marine Mammal Science, 19, 232–239. https://doi.org/10.1111/j.1748-7692.2003.tb01105.x
North Atlantic Marine Mammal Commission (NAMMCO). (1998a). Report of the 5th meeting of the Scientific Committee, Tromsø, Norway. https://nammco.no/topics/scientific-committee-reports/
North Atlantic Marine Mammal Commission (NAMMCO). (1998b). Report of the Management Committee. In NAMMCO Annual Report 1997, NAMMCO, Tromsø, Norway, 63–82. https://nammco.no/topics/annual-reports/
North Atlantic Marine Mammal Commission (NAMMCO). (2013). Report of the 19th Meeting of the Scientific Committee, Tasiilaq, Greenland. NAMMCO, Tromsø, Norway. 641pp. https://nammco.no/topics/scientific-committee-reports/
Natale, A.D. and Notarbartolo-di-Sciara, G. (1994). A review of the passive fishing nets and trap fisheries in the Mediterranean Sea and of the cetacean bycatch. Report of the International Whaling Commission, sp 15, 189–202.
Nelson, D. and Lien, J. (1996). The status of the long-finned pilot whale, Globicephala melas, in Canada. Canadian Field-Naturalist, 110, 511–524.
Notarbartolo-di-Sciara, G. (1990). A note on the cetacean incidental catch in the Italian driftnet swordfish fishery, 1986-1988. Report of the International Whaling Commission, 40, 459–460.
Olson, P.A. (2018). Pilot Whales. In B. Würsig, J.G.M. Thewissen, K.M. Kovacs(eds), Encyclopedia of Marine Mammals, 701–705. Elsevier, 3rd ed. https://doi.org/10.1016/B978-0-12-804327-1.00194-1
Oremus, M., Gales, R., Dalebout, M.L. et al. (2009). Worlwide mitochondrial DNA diversity and phylogeography of pilot whales (Globicephala spp.). Biological Journal of the Linnean Society of London, 98, 729–744. https://doi.org/10.1111/j.1095-8312.2009.01325.x
Ottensmeyer, C.A. and Whitehead, H. (2003). Behavioural evidence for social units in long-finned pilot whales. Canadian Journal of Zoology, 81, 1327–1338. https://doi.org/10.1139/z03-127
Overholtz, W.J. and Waring, G.T. (1991). Diet composition of pilot whales Globicephala sp. and common dolphins Delphinus delphis in the Mid-Atlantic Bight during spring 1989. Fisheries Bulletin, 89, 723–728.
Palka, D. 2020. Cetacean abundance estimates in US northwestern Atlantic Ocean waters from summer 2016 line transect surveys conducted by the Northeast Fisheries Science Center. Northeast Fish. Sci. Cent. Ref. Doc.20-05.
Payne, P.M. and Heinemann, D.W. (1993). The distribution of pilot whales (Globicephala spp.) in shelf/shelf-edge and slope waters of the northeastern United States, 1978-1988. Report of the International Whaling Commission, sp 14, 51–68.
Pierrepont, J.F.D., Dubois, B., Desormonts, S. et al. (2005). Stomach contents of English Channel cetaceans stranded on the coast of Normandy. Journal of Marine Biological Association of the United Kingdom, 85, 1539-1546. http://dx.doi.org/10.1017/S0025315405012762
Pike, D.G, Desportes, G., Gunnlaugsson, T., Mikkelsen, B. and Bloch, D. (2013). Estimates of the relative abundance and trend of pilot whales (Globicephala melas) in the North Atlantic from 1987 to 2007. NAMMCO SC/20/18 for the NAMMCO Scientific Committee. 54pp.
Pike, D.G., Gunnlaugsson, T., Desportes, G. et al. (2019a). Estimates of the relative abundance of long-finned pilot whales (Globicephala melas) in the Northeast Atlantic from 1987 to 2015 indicate no long-term trends. NAMMCO Scientific Publications, 11. https://doi.org/10.7557/3.4643
Pike, D.G., Gunnlaugsson, T., Mikkelsen, B. et al. (2019b). Estimates of the abundance of cetaceans in the central north Atlantic based on the NASS Iceland and Faroese shipboard surveys conducted in 2015. NAMMCO Scientific Publications, 11. https://doi.org/10.7557/3.4941
Pike, D. G., Gunnlaugsson, T., Mikkelsen, B. et al. (2020). Estimates of the Abundance of Cetaceans in the Central North Atlantic from the T-NASS Icelandic and Faroese Ship Surveys Conducted in 2007. NAMMCO Scientific Publications, 11. https://doi.org/10.7557/3.5269
Praca, E., Laran, S., Lepoin,t G. et al. (2011). Toothed whales in the northwestern Mediterranean: Insight into. their feeding ecology using chemical tracers. Marine Pollution Bulletin, 62, 1058–1065. https://doi.org/10.1016/j.marpolbul.2011.02.024
Raga, J.A. and Balbuena, J.A. (1993). Parasites of the long-finned pilot whale, Globicephala melas (Traill, 1809), in European waters. Report of the International Whaling Commission, sp 14, 391–406.
Reijnders, P.J.H. and de Ruiter-Dijkman, E.M. (1995). Toxicological and epidemiological significance of pollutants in marine mammals. In A.S. Blix, L. Walløe and Ø. Ulltang (eds.), Whales, seals, fish and man, 575–587. Amsterdam, Elsevier. https://doi.org/10.1016/S0163-6995(06)80056-9
Rendell, L.E. and Gordon, J.C.D. (1999). Vocal response of long-finned pilot whales (Globicephala melas) to military sonar in the Ligurian Sea. Marine Mammal Science, 15(1), 198–204. http://dx.doi.org/10.1111/j.1748-7692.1999.tb00790.x
Rendell, L.E., Matthews, J.N., Gill, A. et al. (1999). Quantitative analysis of tonal calls from five odontocete species, examining interspecific and specific variation. Journal of Zoology, 249(4), 403–410. https://doi.org/10.1111/j.1469-7998.1999.tb01209.x
Roberts, J., Best, B., Mannocci, L. et al. (2016). Habitat-based cetacean density models for the U.S. Atlantic and Gulf of Mexico. Scientific Reports, 6, 22615. https://doi.org/10.1038/srep22615
Rogan, E., Cañadas, A., Macleod, K. et al. (2017). Distribution, abundance and habitat use of deep diving cetaceans in the North-East Atlantic. Deep Sea Research Part II: Topical Studies in Oceanography, 141, 8–19. https://doi.org/10.1016/j.dsr2.2017.03.015
Rogan, E., Breen, P., Mackey, M. et al. (2018). Aerial surveys of cetaceans and seabirds in Irish waters: occurrence, distribution and abundance in 2015-2017. Dublin, Ireland
Sanderson, K. (1992). Grindadráp: A Textual History of Whaling Traditions in the Faroes to 1900. MPh thesis, University of Sydney, Australia.
Sanderson, K. (1994). Grind – Ambiguity and Pressure to Conform: Faroes whaling and the anti-whaling protest. In M.M.R. Freeman and U.P. Kreter (eds.), Elephants and Whales: Resources for whom? 187–201. Philadelphia, USA: Gordon and Breach Science Publishers.
Santos, M.B., Monteiro, S.S., Vingada, J.V. et al. (2014). Patterns and trends in the diet of long-finned pilot whales (Globicephala melas) in the northeast Atlantic. Marine Mammal Science, 30, 1–19. http://dx.doi.org/10.1111/mms.12015
Senigaglia, V. and Whitehead, H. (2012). Synchronous breathing by pilot whales. Marine Mammal Science, 28, 213–219. http://dx.doi.org/10.1111/j.1748-7692.2011.00465.x
Senigaglia, V., de Stephanis, R., Verborgh, P. and Lusseau, D. (2012). The role of synchronized swimming as affiliative and anti-predatory behavior in long-finned pilot whales. Behavioural Processes, 91, 8–14. http://dx.doi.org/10.1016/j.beproc.2012.04.011
Sergeant, D.E. (1962). The biology of the pilot or pothead whale Globicephala melaena (Traill) in Newfoundland waters. Bulletin of the Fisheries Research Board Canada, 132, 1–84.
Sergeant, D.E. and Fisher, H.D. (1957). The smaller cetaceans of eastern Canadian waters. Journal of the Fisheries Research Board of Canada, 14, 83–115. https://doi.org/10.1139/f57-003
Siemann, L. (1994). Mitochondrial DNA sequence variation in North Atlantic long-finned pilot whales, Globicephala melas. Ph.D. Thesis, Massachusetts Institute of Technology/Woods Hole Oceanographic Institution. https://doi.org/10.1575/1912/4942
Sigurjónsson, J. and Vikingsson, G.A. (1997). Seasonal abundance of and estimated food consumption by cetaceans in Icelandic and adjacent waters. Journal of the Northwest Atlantic Fishery Science, 22, 271–287. https://doi.org/10.2960/J.v22.a20
Sigurjónsson, J., Víkingsson, G. and Lockyer, C. (1993). Two mass strandings of pilot whales (Globicephala melas) on the coast of Iceland. Report of the International Whaling Commission, sp 14, 407–423.
Sivle, L.D., Kvadsheim, P.H., Fahlman, A. et al. (2012). Changes in dive behavior during naval sonar exposure in killer whales, long-finned pilot whales, and sperm whales. Frontiers in Physiology, 3, 400. http://dx.doi.org/10.3389/fphys.2012.00400
Smith, T.D., Payne, P.M., Heinemann, D., Waring, G. and Langre, A. (1990). Simultaneous fishery resources and seabird and cetacean sighting surveys. North Atlantic Studies, 2, 90–101.
Sonne, C., Dam, M., Leifsson, P.S. and Dietz, R. (2010). Liver and renal histopathology of North Atlantic long- finned pilot whales (Globicephala melas) contaminated with heavy metals and organochlorine compounds. Toxicological and Environmental Chemistry, 92, 969–985. http://dx.doi.org/10.1080/02772240903187221
Spitz, J., Cherel, Y., Bertin, S. et al. (2011). Prey preferences among the community of deep-diving odontocetes from the Bay of Biscay, Northeast Atlantic. Deep Sea Research I: Oceanographic Research Papers, 58, 273–282. http://dx.doi.org/10.1016/j.dsr.2010.12.009
Spitz, J., Trites, A.W., Becquet, V. et al. (2012). Cost of living dictates what whales, dolphins and porpoise eat: the importance of prey quality on predator foraging strategies. Plos ONE, 7(11), e50096. http://dx.doi.org/10.1371/journal.pone.0050096
Steiner, W. W. (1981). Species-specific differences in pure tonal whistle vocalizations of five western North Atlantic dolphin species. Behavioral Ecology and Sociobiology, 9, 241–246. https://doi.org/10.1007/BF00299878.
Steuerwald, U., Weihe, P., Jørgensen, P.J. et al. (2000). Maternal seafood diet, methylmercury exposure, and neonatal neurologic function. Journal of Pediatrics, 136(5), 599–605. http://dx.doi.org/10.1067/mpd.2000.102774
Taylor, B.L., Baird, R., Barlow, J. et al. (2008). Globicephala melas. IUCN Red List of Threatened Species.
Taruski, A.G. (1979). The whistle repertoire of the North Atlantic pilot whale (Globicephala melaena) and its relationship to behaviour and environment. In H.E. Winn. and B.L. Olla (eds.), Behaviour of marine mammals, 345–368. New York: Plenum Press. https://doi.org/10.1007/978-1-4684-2985-5_10
Tilbury, K.L., Adams, N.G., Krone, C.A. et al. (1999). Organochlorines in Stranded pilot whales (Globicephala melaena) from the Coast of Massachusetts. Archives of Environmental Contamination and Toxicology, 37, 125–134. https://doi.org/10.1007/s002449900497
Traill, T.S. (1809). Description of a new species of whale, Delphinus melas. In a letter from Thomas Stewart Traill, M.D. to Mr. Nicholson. Journal of Natural Philosophy, Chemistry, and the Arts, 22, 81–83.
Verborgh, P. (2015). Demografía y estructura de las poblaciones de calderones comunes (Globicephala melas) en el Mediterráneo español. PhD Thesis, Universidad de Las Palmas de Gran Canaria.
Verborgh, P. and Desportes, G. 2021. Long-finned pilot whale Globicephala melas (Traill 1809). In K. Hackländer and F.E. Zachos (eds.), Handbook of the Mammals of Europe, xxx-xxx. Springer.
Verborgh, P., Gauffier, P., Esteban, R., et al. (2016). Conservation status of long-finned pilot whales, Globicephala melas, in the Mediterranean Sea. In G. Notarbartolo di Sciara, M. Podestà, B.E. Curry (eds.), Advances in Marine Biology 75: Mediterranean Marine Mammal Ecology and Conservation, 173–203. Oxford: Academic P. Elsevier.
Vester, H., Hallerberg, S., Timme, M. and Hammerschmidt, K. (2017). Vocal repertoire of long-finned pilot whales (Globicephala melas) in northern Norway. Journal of the Acoustical Society of America, 141, 4289–4299. https://doi.org/10.1121/1.4983685
Visser, F. (2014). Moving in concert: social and migratory behaviour of dolphins and whales in the North Atlantic Ocean. PhD thesis. Institute for Biodiversity and Ecosystem Dynamics. University of Amsterdam. http://dare.uva.nl/record/472565
Visser, F., Miller, P.J.O., Antunes, R.N. et al. (2014). The social context of individual foraging behaviour in long-finned pilot whales (Globicephala melas). Behaviour, 51(10), 1453–1477. http://dx.doi.org/10.1163/1568539X-00003195
Visser, F., Kok, A.C.M., Oudejans, M.G. et al. (2017) Vocal foragers and silent crowds: context-dependent vocal variation in Northeast Atlantic long-finned pilot whales. Behavioral Ecology and Sociobiology, 71, 170. https://doi.org/10.1007/s00265-017-2397-y
Waring, G.T., Josephson, E., Maze-Foley, K. and Rosel. P.E. (eds.). (2012). U.S. Atlantic and Gulf of Mexico Marine Mammal Stock Assessments – 2011. NOAA Technical Memorandum NMFS-NE-221. 319pp. (G. melas, p. 58-70). Available at http://www.nmfs.noaa.gov/pr/pdfs/sars/ao2011.pdf
Waring, G.T., Josephson, E., Fairfield, C.P., Maze-Foley, K. (eds.). (2007a). U.S. Atlantic and Gulf of Mexico Marine Mammal Stock Assessments – 2006. NOAA Technical Memorandum NMFS NE-201. 378pp. http://nefsc.noaa.gov/publications/tm/tm201/
Waring, G.T., Josephson, E., Fairfield, C.P., Maze-Foley, K. (eds.). (2007b). U.S. Atlantic and Gulf of Mexico Marine Mammal Stock Assessments – 2007. NOAA Technical Memorandum NMFS NE-205. 415pp. http://www.nefsc.noaa.gov/publications/tm/tm205/pdfs/96LongFinPilotW.pdf
Waring, G.T., Palka, D.L., Clapham, P.J. et al. (1999). U.S. Atlantic and Gulf of Mexico marine mammal stock assessments – 1999. NOAA Technical Memorandum NMFS NE-153.
Weilgart, L.S. and Whitehead, H. (1990). Vocalizations of the North Atlantic pilot whales (Globicephala melas) as related to behavioural context. Behavioral Ecology and Sociobiology, 26, 399–402. http://dx.doi.org/10.1007/BF00170896
Weihe, P. and Joensen, H. D. F. (2012). Dietary recommendations regarding pilot whale meat and blubber in the Faroe Islands. International Journal of Circumpolar Health, 71, 18594. http://dx.doi.org/10.3402/ijch.v71i0.18594
Weihe, P. and Joensen, H.D. (2008). Recommendations to the government of the Faroe Islands concerning the pilot whale. (English translation released 1 December 2008.) Tórshavn: Landslægen.
Weihe, P., Grandjean, P., Debes, F. and White, R. (1996). Metals and PCB’s from pilot whales. The Science of the Total Environment, 186, 141–148. http://dx.doi.org/10.1016/0048-9697(96)05094-2
Weisbrod, A.V., Shea, D., Moore, M.J. and Stegeman, J.J. (2000). Bioaccumulation patterns of polychlorinated biphenyls and chlorinated pesticides in northwest Atlantic pilot whales. Environmental Toxicology and Chemistry, 19, 667–677. http://dx.doi.org/10.1002/etc.5620190319
Weisbrod, A.V., Shea, D., Moore, M.J. and Stegeman, J.J. (2001). Species, tissue and gender-related organochlorine bioaccumulation in white-sided dolphins, pilot whales and their common prey in the Northwest Atlantic. Marine Environmental Research, 51, 29–50. http://dx.doi.org/10.1016/S0141– 1136(00)00032-5
Williamson, K. (1970). The Atlantic Islands. A study of the Faroe life and scene. Routledge and K. Paul London. 385pp.
Zachariassen, P. (1993). Pilot whale catches in the Faroe Islands, 1709-1992. Report of the International Whaling Commission, sp 14, 69–88.
Zwamborn, E.M.J and Whitehead, H. (2017). Repeated call sequences and behavioural context in long-finned pilot whales off Cape Breton, Nova Scotia, Canada. Bioacoustics, 26, 169–183. https://doi.org/10.1080/09524622.2016.1233457
Read more about pilot whales in the links below:
Faroe Islands, Ministry of Fisheries – Whales and Whaling in the Faroe Islands
Greenland Institute of Natural Resources – Pilot Whale
Norwegian Polar Institute – Pilot Whale
International Whaling Commission (IWC) – Pilot Whale
IUCN Red List – Long-finned Pilot Whale Status Report
Convention on the Conservation of Migratory Species of Wild Animals (CMS) – Long-finned Pilot Whale
Fourth Report of the UK under Article 17 of the European Community Directive on the Conservation of Natural Habitats and of Wild Flora and Fauna: Conservation Status Assessment for Species – Long-finned Pilot Whale
NOAA Fisheries – Long-finned Pilot Whales
University of Michigan Museum of Zoology – Animal Diversity Web – Long-finned Pilot Whale
General Resources:
Environmental Data on Terrestrial and Marine Ecosystems in the Faroe Islands (ENVOFAR)
Atlas of Cetacean Distribution in the North-West European Waters
AMAP – Arctic Monitoring and Assessment Programme (Arctic Council)
CAFF – Conservation of Arctic Flora and Fauna (Arctic Council)