Humpback Whale
Humpback Whale
Latest update: February 2022, reviewed by NAMMCO’s Scientific Committee
The humpback whale (Megaptera novaeangliae) is a baleen whale from the family Balaenopteridae. This family includes species such as the blue (Balaenoptera musculus), minke (B. acutorostrata) and fin (B. physalus) whales. They are characterised by their large size, ventral grooves that allow the mouth to expand, streamlined shape, high swimming speed, and relatively short baleen plates. Humpback whales arch their backs at the sea surface when initiating a dive, which is the origin of the common name for the species. The genus name Megaptera means “large wing” in Greek and describes the very large pectoral fins, which are about 1/3 the body length – the largest of any whale. The pectoral fins are mostly white in colour and easily distinguish humpbacks from other large whales. Males and females are similar in body form, coloration, and size, although females are slightly larger than males.
ABUNDANCE
Estimates indicate that there are at least 35,000 humpback whales in the North Atlantic.
DISTRIBUTION
Humpback whales are found worldwide, except for in the very high Arctic. In the North Atlantic they migrate between breeding grounds in the tropics (the Caribbean) and feeding grounds as far north as northern Norway.
RELATION TO HUMANS
There has been a long history of exploitation of the species by both subsistence and commercial whaling. Today in the North Atlantic they continue to be hunted in very low numbers in West Greenland and St Vincent and the Grenadines
They are also a popular target for whale-watching operations worldwide.
CONSERVATION AND MANAGEMENT
Where data is available, the indication is that populations of humpback whales are increasing. In the NAMMCO countries, for example, increases of 12% per year off Iceland between 1986-2001 and 9.4% per year off West Greenland between 1984-2007 have been documented.
International management occurs through the International Whaling Commission (IWC) and NAMMCO. NAMMCO provides scientific advice on stock status, sustainable takes, and proposals for conservation and management to its member governments. The IWC gives advice on the sustainability of quotas.
The species is listed as ‘Least Concern and increasing’ on both global and regional European IUCN Red List for the latest assessments in 2018 and 2023, respectively. The species is ‘Least Concern’ on both Norwegian and Icelandic national red list.

© Fernando Ugarte
Scientific name: Megaptera novaeangliae
Faroese: Kúlubøka
Greenlandic: Qipoqqaq
Icelandic: Hnúfubakur
Norwegian: Knølhval
Danish: Pukkelhval
English: Humpback whale
Size
Maximum length is around 17 m, maximum weight about 36 tonnes, although the average size is smaller than this. Females grow to a slightly larger size than males. Calves are about 6 m and 1.8 tonnes at birth.
Productivity
Humpback females have 1 calf every 2–3 years from an age of around 4–7 years.
Lifespan
They can live up to about 70 years of age.
Migration
Humpback whales migrate annually between tropical breeding and mating areas occupied in the late winter and spring, to high latitude feeding areas in the summer, autumn and early winter. The annual return trip between feeding and breeding areas can exceed 15,000 km.
Feeding
They feed mainly on euphausiids (krill) and small schooling fish.
General Characteristics
The humpback whale (Megaptera novaeangliae) is a baleen whale from the family Balaenopteridae. This family includes the blue (Balaenoptera musculus), minke (B. acutorostrata) and fin (B. physalus) whales. Balaenopteridae, or commonly called ‘rorquals’, are characterised by their large size (the blue whale is the largest animal that has ever lived), ventral grooves allowing their mouth to expand widely when engulfing food, a streamlined shape, high swimming speed, and relatively short baleen plates. Humpback whales arch their backs at the sea surface when initiating a dive, which is the origin of the common name for the species. The genus name Megaptera means “large wing” in Greek and describes the very large pectoral fins, which are about 1/3 the body length – the largest of any whale.
Appearance
Humpback whales are variable in colouration, but are usually dark grey or nearly black dorsally, with some white patterning on their flukes and pectoral fins. In the North Atlantic, pectoral fins tend to be white as opposed to those of the North Pacific, which are generally black (Clapham 2018). The white markings, combined with nicks and gouges on the flukes, are individually distinctive and are frequently used to identify individual animals (Smith et al. 1999). The ventral side is much lighter in colour and sometimes mostly white. The ventral grooves run from the bottom part of their rostrum (nose/beak) down about half the length of their body. The head and lower jaw are covered in knobbly hair follicles called tubercles and also frequently host a heavy growth of barnacles.

Humpback whale showing the knobby “tubercles” on the rostrum (nose). © Fernando Ugarte
Reproduction
Humpback whales reach a maximum length of about 17 m and a maximum weight of about 36 tonnes, although the typical length is 14-15m (Clapham and Mead, 1999). Females grow to a slightly larger size than males (Bettridge et al. 2015). Calves are born after a gestation of 11–12 months and are around 6 m and 1.8 tonnes at birth. Calves nurse from 6 months up to a year and are independent after about one year.
Humpback whales reach sexual maturity at 4–7 years of age and give birth to a single calf every 2–3 years on average (IWC 2002, NAMMCO 2009). The only known predator of humpbacks is the killer whale (Orcinus orca), which predominantly take calves in the North Atlantic (Ford & Reeves 2008).
Behaviour
Humpback whales are long-distance travellers and make the longest annual migrations of any mammal. In the North Atlantic, they migrate annually between tropical breeding and mating areas in the late winter and spring, to high latitude feeding areas in the summer, autumn and early winter. The annual return trip between feeding and breeding areas can exceed 15,000 km. Despite their long migrations, humpback whales are not fast swimmers compared to other members of their family. They normally travel 2–12 km/h and cover up to 200 km per day during their migration period (Heide-Jørgensen & Laidre 2007, Ford & Reeves 2008).
Humpback whales are a favourite of whale-watchers because they are very active at the surface, often breaching, rolling, slapping the surface with their pectoral fins or fluke and gulping prey. They also often engage in group feeding behaviour.
Humpbacks make a wide variety of sounds but are known for their complex and characteristic “songs” sung primarily on breeding grounds, which last up to 30 minutes and are distinctive to stocks (Payne & McVay 1971; Bettridge et al. 2015).
Listen to humpback whale songs on their breeding grounds in the Silver Banks
DID YOU KNOW?
Humpback Whales Have Inspired New Wind Turbine Technology
The humpback manages tighter turns than any other baleen whale thanks to the tubercles (bumps) on the leading edges of its flippers. Research found that humpback whale flippers have an angle of attack up to 40 percent steeper than that of a smooth flipper before stall (loss of lift) occurs. Tubercles change the distribution of pressure on the flippers so that some parts stall before others. Therefore, different parts of the flipper stall at different angles of attack, which gives the whale more freedom to attack at higher angles.
Prototypes of wind turbines blades mimicking humpback whales’ pectoral fins have been created. Tests show that the turbines with tubercles generate the same amount of power at 16 km per hour that conventional turbines generate at 27 km per hour. Tubercles on the blade’s leading edge enable it to capture more energy from the wind, but also reduce noise and increase its stability.
You can read more about this interesting biomimetic research here.
- © Thara58/Pixabay
- © WhalePower
LIFE HISTORY AND ECOLOGY
As members of the family Balaenopteridae, humpback whales are baleen whales. Baleen is composed of plates of keratin – a fibrous structural protein that also is found in fingernails, claws and hair. These plates hang down from the upper jaw, taking the place of teeth in baleen whales. Baleen plates are fringed by fine hairs that form a fibrous filter when the plates are extended. Humpback whales have between 270 and 400 baleen plates on each side of their jaw (Clapham 2018). Baleen whales feed by engulfing a large quantity of water that contains their prey, unfolding their ventral grooves to expand their mouth cavity, then expelling the water through the baleen. The baleen filter out the water and maintain the prey items, which are then swallowed.
The humpback whale is a medium-sized baleen whale, smaller than the blue, fin, right or bowhead whales but larger than sei or minke whales. Calves are born in tropical waters after a gestation period of 11–12 months, typically in mid-winter (Clapham 2018). Mating occurs in the same area, however, females who have given birth do not usually mate in the same year. This leads to a calving interval of 2–3 years. Males and females reach sexual maturity at an age of 5–10 years (Clapham 2018), and individuals may live up to about 70 years. Calves rely on their mothers for milk for about the first six months of their lives, and are weaned

© NOAA
and independent at one year of age (IWC 2002, Bettridge et al. 2015, Clapham et al. 2003).
Humpback whales travel to the tropical breeding grounds in most years to breed. Males are polygynous, meaning that they may mate with several females in a single season. During the breeding season, males often compete aggressively, frequently butting heads or ramming one another, or hitting each other with their flukes or flippers. The tubercles (the hard, knobbly growths on the head, lower jaw, fins and flukes) may serve as weapons in this aggression. They are often encrusted with barnacles–a single whale may carry as much as 450 kg of barnacles!–and the hard, sharp barnacle encrustation may serve to enhance the effect of a blow, whether in competition with another male or in defence against a predator (Ford & Reeves 2008).
During most times of the year, humpback whales are found in small groups of 2–5 animals. Sometimes larger groups form temporarily to engage in cooperative feeding aggregations. Groups of up to 95 animals have been spotted off West Greenland, but the function of these very large groups is unknown (Heide-Jørgensen et al. 2008).
DIET AND FEEDING
Humpback whales have a diverse diet and exhibit great flexibility and variation in their feeding behaviour. They aggregate in areas where prey has naturally been conc

Humpback whales feeding off the coast of Norway. © Fernando Ugarte
entrated by currents and upwelling, or by spawning and migratory behaviour. Their manoeuvrability and social cooperation further concentrate and trap prey, making their feeding more efficient.
In the North Atlantic, humpback whales feed mainly on euphausiids (krill) and small schooling fish such as capelin (Mallotus villosus), Atlantic herring (Clupea harengus), sand eels (Ammodytes spp.), and mackerel (Scomber spp.). Their diet varies by season and feeding ground, depending on the availability of suitable prey. In the Gulf of Maine, for example, humpback whales feed on spawning herring, which form large dense schools at night (Gong et al. 2014). Off Greenland and Iceland, however, they feed primarily on euphausiids, capelin and sand eels (Heide-Jørgensen & Laidre 2007, Heide-Jørgensen & Simon 2007, Magnusdóttir et al. 2014).
Humpback whales appear to track the movements of their prey and change their distribution in response to changes in the abundance and distribution of prey species. For example, the distribution of humpbacks has changed greatly around Iceland and northern Norway in recent years, apparently in response to changes in the distribution of capelin and herring (Víkingsson et al. 2015).
Feeding behaviour
Feeding dives may last for as long as 30 minutes, although they are usually much shorter (Heide-Jørgensen & Laidre 2007). Feeding depth varies with the distribution of the prey but seldom exceeds 200 m. The deepest recorded dive depth was 616m, reached by a whale in the east of the Bellona plateau (Derville et al. 2020).
Humpback whales have a much more varied repertoire of feeding behaviours than most other baleen whales. Humpbacks forage alone or in groups. The most common feeding mode is “lunge feeding” either at depth during a dive or at the surface, in which the whale accelerates rapidly forward (lunges), opening its mouth, expanding its buccal cavity by stretching out the ventral grooves, and engulfing a volume of prey-laden water that can exceed its own weight (Simon et al. 2012). The whale then expels this water through its baleen to filter out the water and maintain the prey, which is then swallowed.

Humpback whale bubble feeding © Christine Khan/NOAA
Another feeding strategy for some humpback whale populations is to blow walls or nets of bubbles that they use to corral and herd schools of fish. They often do this in small groups, cooperating to concentrate their prey before engulfing it. This frequently results in spectacular surface displays, with groups of humpbacks breaching the surface as they engulf schooling fish, amidst a cloud of bubbles (Bettridge et al. 2015). These are behaviours that must be learned and practiced, implying a relatively high degree of cultural transmission. Humpbacks may be one of the only cetaceans to engage in this form of tool use.
Feeding activity often varies with time of day, following the vertical migrations of their prey. In some areas, humpbacks dive to the bottom and roll repeatedly, likely startling bottom dwelling fish into the water column where they can then be engulfed. However, much feeding is done near the surface, which increases the appeal of this species for whale watchers.
DID YOU KNOW?
Whale Lice Can Tell Us About the Social Network of Humpback Whales
The seven identified humpback whale stocks in the Southern Hemisphere all travel to Antarctic waters during the summer. While scientists have good documentation on these seasonal migration patterns, little is known about the summer activities and whether the different stocks interact with one another. As satellite data does not provide much insight into this, Tammy Iwasa-Arai, a PhD researcher, chose to investigate this subject through a different lens: parasites, in particular whale lice.
Iwasa-Arai focused on Cyamus boopis, an amphipod that feeds off humpbacks skin. This species only parasitises humpbacks and sticks with them for their entire lives, making it an ideal data gatherer. Having collected lice off stranded whales in different parts of the Southern Hemisphere, the researchers sequenced their DNA to determine the lice’s population structure. In doing so, they sought to learn more about the interactions of the different humpback whales stocks these lice had infested: the greater the genetic similarity between lice, the more interactions across different humpback stocks. The results were remarkable, the western South Atlantic stock had more interactions with the western South Pacific stock than with its neighbouring eastern South Atlantic stock. This demonstrates that the behaviour of the humpback whale is complex and still poorly understood today.
You can read the whole study by Iwasa-Arai et al. here.

©Feng Yu/Shutterstock.com
Breeding grounds
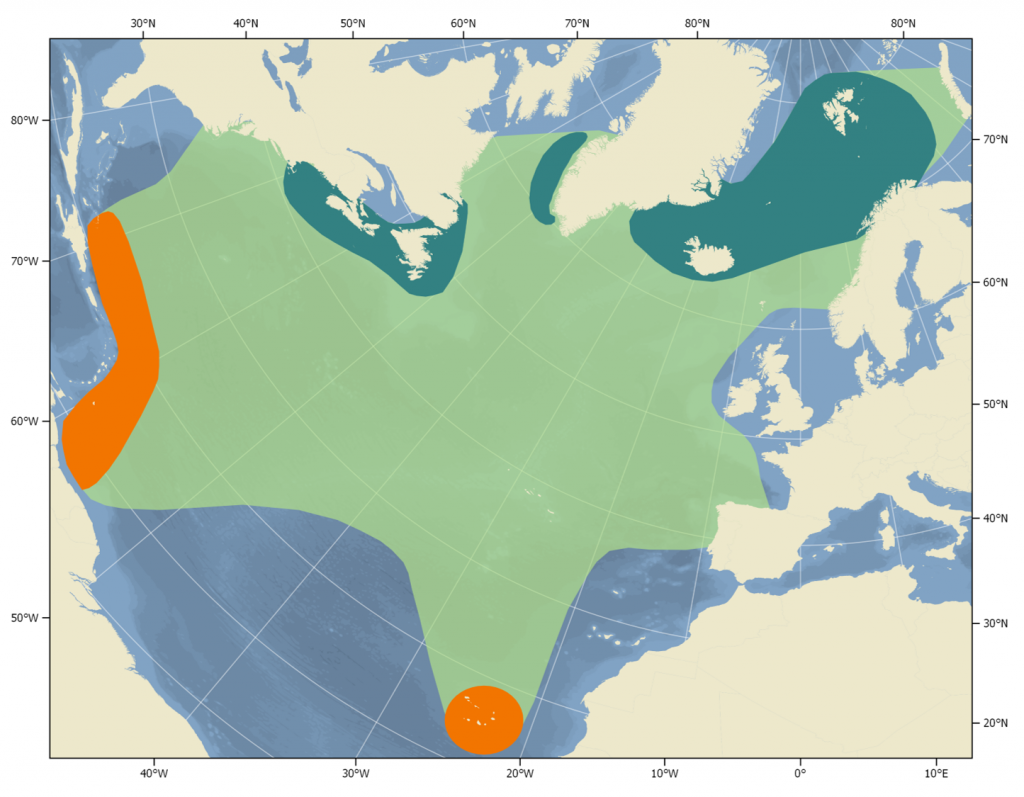
Distribution of humpback whales in the North Atlantic (light green). Orange indicates breeding & calving areas, dark green indicates feeding areas.
Humpback whales are the mammalian champions of migration, travelling up to 15,000 km per year between their tropical breeding/mating area and their high latitude feeding grounds. In the Northern Hemisphere, they begin to arrive at the breeding grounds in February and most are gone again by the end of April (Stevick et al. 2016). Animals from the more distant feeding grounds off Northern Norway and Iceland tend to arrive slightly later (Stevick et al. 1999). Breeding grounds are found in the tropics and are usually in fairly shallow waters, close to shore, or associated with offshore reefs (IWC 2002). Humpbacks spend the remainder of the year at northern feeding grounds, which range in latitude from 43°N (Gulf of Maine) to the north Norwegian Sea (75°N). Feeding grounds are typically characterised by areas where water masses mix, which causes upwelling and high productivity. This leads to high concentrations of pelagic crustaceans and small fish, which enables the whales to feed effectively.
Humpback whales are largely faithful to their own feeding and breeding areas, as indicated by both photographic and genetic evidence (Smith et al. 1999). However, some exchange between breeding areas has been observed (Stevick et al. 2016). They also typically exhibit site fidelity on a smaller spatial scale, with some animals, for example, occupying the same bay in Greenland year after year (Boye et al. 2010).

© Fernando Ugarte
Breeding grounds in the North Atlantic
West Indies
Two humpback whale breeding grounds are known with certainty in the North Atlantic. In terms of whale numbers, by far the largest is in the West Indies. Whales from most North Atlantic feeding grounds migrate here to breed (Smith et al. 1999). The area utilised by humpbacks extends from Cuba in the northwest and south to Venezuela. The largest concentrations of breeding animals are found on the Silver and Navidad Banks near the Dominican Republic, with much lower numbers in Samana Bay (Dominican Republic), off the northwest coast of Puerto Rico, and around the Virgin Islands and the eastern Antilles (Reilly et al. 2008, IWC 2002, Smith et al. 1999).
Whaling records indicate that, historically, the principal Caribbean breeding ground occurred further south in the Windward Islands, an area that has few breeding whales today (Smith & Reeves 2011). However, recent satellite tagging studies have shown that some whales make extensive inter-island movements in the area (Kennedy et al. 2013). The breeding area is characterised by relatively shallow water (<200 m), low slope and warm water temperatures between 24–28 °C (Stevick et al. 2016). Humpbacks begin to arrive in this area in early February (peaking in late February) and by the end of March, few whales remain (Stevick et al.2016).
Cape Verde Islands
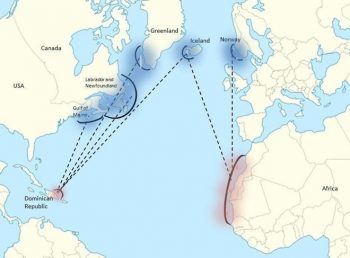
Migration patterns of North Atlantic humpback whales, where blue shows summer foraging grounds and red indicates the general area of breeding grounds. Figure from: Mackay 2015.A second breeding area is found around the Cape Verde Islands off West Africa. This area hosts few whales today (Wenzel et al. 2009), but whaling records suggest that substantial numbers occurred here in the past (Smith and Reeves 2011). Only whales from the Icelandic and Norwegian feeding areas are known to use this area, although most whales from these feeding areas use the West Indies ground (Wenzel et al. 2009). This breeding ground is occupied from the beginning of March, a few weeks later than the West Indies ground, with peak occupation occurring in early April (Stevick et al. 2016).
Other breeding grounds are suspected but not yet confirmed. Humpback whale song has been recorded year-round in the Norwegian Sea (Martin et al. 2021), and animals with full-term foetuses were taken by whaling operations in northern Norway in the late 19th and early 20th centuries. This suggests that a breeding area may exist in this area (IWC 2002, Smith & Pike 2009). A third breeding ground off West Africa has also been suggested (Punt et al. 2007). There may also be finer scale partitioning of the West Indies ground, with whales from different feeding grounds using separate areas within the overall breeding area (Stevick et al. 2016).
Variability and changes in migratory behaviour
While humpback whales are typically faithful to their natal breeding ground (returning to breed in the area in which they were born) (IWC 2002), four individuals were recently identified at the West Indies and at Cape Verde breeding grounds in different years, suggesting an unexpected degree of flexibility in the migratory behaviour of these animals (Stevick et al. 2016).
Not all humpback whales make the return trip from the feeding grounds to the breeding grounds every year. Some linger on the Icelandic feeding ground throughout the entire winter, following the migrating capelin (Mallotus villosus) (Magnusdóttir et al. 2014). Humpback whales have also been observed throughout the winter off Greenland, Norway and the British Isles (IWC 2002, Heide-Jørgensen & Laidre 2007, Smith & Pike 2009).
There is also some evidence that the migratory behaviour of humpback whales has changed over time. Whaling records indicate that the principal Caribbean breeding ground occurred much further south in the Windward Islands in the past. This is an area that has few breeding whales today (Smith & Reeves 2011). Currently, the main breeding ground lies further north off the Dominican Republic and Puerto Rico (Smith et al. 1999). A large number of whales were previously harvested from the Cape Verde breeding ground, which is an area visited by few whales today (Smith & Reeves 2011, Reilly et al. 2008, Wenzel et al. 2009).
In 2011, humpback whales begun to occur in the fjords of Northern Norway, where they had been completely absent for decades. These whales appear to follow the migration of the Norwegian spring-spawning herring along the coast of Northern Norway. The migratory behaviour of humpback whales therefore seems to have a degree of flexibility. With some animals apparently not migrating in some years, and breeding and feeding areas changing slightly over decades, it seems that migratory patterns have the potential to change in response to prey distribution or other factors.
Feeding grounds
Six discrete feeding grounds are recognised in the North Atlantic. These tend to be separated from one another by gaps of hundreds of kms in which few humpback whales occur (Vigness-Raposa et al. 2010). Three feeding stocks occur off Eastern North America: the Gulf of Maine/Scotian Shelf, the Gulf of St. Lawrence, and Newfoundland/Labrador. In the Central Atlantic, humpbacks feed off West Greenland and on the Icelandic continental shelf. The easternmost feeding stock occurs off Northern Norway, especially around Bear and Jan Mayen Islands (IWC 2002, Reilly et al. 2008, Smith & Pike 2009).
Scattered humpback whale sightings have been reported along the Mid-Atlantic Ridge outside of the breeding season, which suggests that this may be another possible feeding ground (Smith & Pike 2009). They may, however, also be animals in transit from the Caribbean breeding ground to the Norwegian or Icelandic feeding grounds (Kennedy et al. 2013). Humpback whales occur in considerable numbers off East Greenland in some years, which may also be another feeding ground or possibly animals from the West Greenland or Iceland grounds (NAMMCO 2017).
Oceanographic conditions
Feeding grounds typically coincide with coastal areas where water masses mix, which causes upwelling and leads to high productivity. This results in high concentrations of crustaceans and pelagic schooling fish, which enables the whales to feed effectively (Víkingsson et al. 2015). The documented feeding grounds range in latitude from 42° (Gulf of Maine) to 75° N (Northern Norway) and therefore encompass a wide variety of marine ecosystems, ranging from temperate to low Arctic. Humpback whales generally do not enter ice-filled waters and are therefore largely absent from the Canadian Arctic, although sightings have been reported there in recent years, probably in response to reductions in sea ice and changing distributions of prey due to climate change (Higdon & Ferguson 2011).
North Atlantic Stocks
To a biologist, stock is a term used to describe a subpopulation of a species that is largely reproductively isolated from others. As a result of this reproductive isolation, stocks can usually be differentiated genetically if they have been isolated for a sufficient length of time. Other means of stock differentiation include morphometrics (body size and shape), concentrations of pollutants or rare elements in tissues, behaviour (including vocal dialects), and patterns of seasonal movement. A key feature of this concept is that the hunting and possible depletion of one stock should have little or no effect on a neighbouring stock.
Genetic and photo-identification studies
There has been little difference detected in humpback whale nuclear DNA among North Atlantic feeding areas. Whales from the Eastern Atlantic feeding grounds could, however, be distinguished from some Western Atlantic groups (IWC 2002, Wenzel et al. 2009). Furthermore, whales from all six feeding grounds can be distinguished from one another using mitochondrial DNA (Larsen et al. 1996, IWC 2002). Mitochondrial DNA is passed directly from mother to offspring, rather than sexually as is the case for nuclear DNA. Genetic analysis therefore suggests that humpback whales tend to use the same feeding grounds throughout their lives (leading to the differences in mitochondrial DNA) but mix with animals from other feeding grounds when mating (explaining the lack of differentiation in nuclear DNA). Such “maternally directed site fidelity” is a pattern seen in several cetacean species, including beluga and narwhal.
Photo-identification studies (in which individual whales are identified and re-sighted based on the markings on their tail flukes), suggest that humpback whales from all feeding grounds mix at the West Indies breeding ground (IWC 2002, Reilly et al. 2008). In contrast, only whales from the Norwegian and Icelandic feeding areas have been traced to the Cape Verde breeding ground (Wenzel et al. 2009). Genetic studies suggest that relatively large proportions of the Icelandic and Norwegian whales do not breed in the West Indies, numbering far more than can be accounted for by the very low numbers found on the Cape Verde breeding ground (Punt et al. 2007, Wenzel et al. 2009). Again this suggests the existence of at least one more breeding ground, possibly in the southern Norwegian Sea or off West Africa (Punt et al. 2007, Smith & Pike 2009).
Conclusion
There is so far no direct evidence from photo-ID or tagging studies of any exchange of whales between feeding grounds. Recently, however, four individuals have been identified in both the West Indies and Cape Verde breeding grounds in different years (Stevick et al. 2016). This suggests that humpbacks are not always faithful to their natal breeding ground. This would also help explain the lack of discrimination in nuclear DNA, if whales are occasionally moved between breeding grounds.
Taken together, the genetic and photo-ID evidence, along with the known migratory patterns of humpback whales, suggest that stocks can be defined by the breeding and feeding grounds of a particular group. This means that whales from the six North Atlantic feeding grounds that breed in the West Indies comprise separate stocks. Additionally, those from the Icelandic and Norwegian areas that breed at Cape Verde or some unknown breeding ground would also be separate stocks, leading to a total of at least 8 North Atlantic stocks of humpback whales. Other feeding grounds may occur off East Greenland (NAMMCO 2017) and along the mid-Atlantic Ridge (Smith and Pike 2009), which would suggest the possibility of additional stocks.
Current Abundance and Trends
Counting humpback whales
Humpback whales are typically counted using line transect surveys, either from a plane or a ship (e.g. Paxton et al. 2009; Pike et al. 2019, 2020a,b; Leonard & Øien 2020a,b).
Since humpback whales can be individually identified by the unique markings on their flukes, population size can also be estimated using “mark-recapture” methodologies. A sample of whales is identified (“marked”) by photographing their flukes. An alternative method of identifying or marking whales is to use a biopsy dart to collect tiny skin samples and then conducting a genetic analysis to identify individuals. A second sample of marked individuals (either by fluke markings or genetics) is “recaptured” or collected some time (e.g. one year) later. If all assumptions are met, the proportion of marked whales in the second recaptured sample should equal the proportion of marked whales in the total population. Smith et al. (1999) applied mark-recapture methodology to the entire North Atlantic basin, collecting fluke photographs and genetic identifications at the West Indies breeding ground and all known North Atlantic feeding grounds over two consecutive years, to derive an abundance estimate for the entire North Atlantic.
Feeding ground estimates
The most recent abundance estimates from North Atlantic feeding grounds within the NAMMCO area are shown in the table below. The aerial survey estimates from East and West Greenland are corrected for all known biases (Hansen et al. 2018). The ship survey estimates from Iceland, the Faroe Islands and Norway are corrected for whales missed by the observers (perception bias) but not for whales that were submerged during the passage of the ship (availability bias); however, this latter form of bias is expected to be small for humpback whales surveyed by ship.
The Icelandic and Norwegian feeding grounds host the largest stock components, likely exceeding 10,000 whales each. Estimates of a similar magnitude have been derived from surveys conducted in these areas from 2002 to 2015 (Pike et al. 2019; Leonard & Øien 2020a, b). Recent abundance off East Greenland was surprisingly high. Taken together with the Icelandic surveys, also conducted in 2015, and correcting for the small overlap between the survey areas, indicates an abundance of 13,916 (95% CI: 9,590–20,193) in the area between Iceland and Greenland (Pike et al. 2019). Altogether, recent estimates indicate that over 25,000 humpback whales occupy the feeding grounds off Norway, the Faroe Islands, Iceland and Greenland during the summer months.
Elsewhere in the North Atlantic, summer abundance in the Gulf of Maine and Scotian Shelf has been estimated to be between 1,000 and 2,000 animals in most years (Clapham et al. 2003; Palka 2012; Lawson & Gosselin 2018). A fully-corrected (i.e., for both perception and availability biases) aerial survey estimate indicated an abundance of 8,439 (CV=0.49) off the Canadian coasts of Newfoundland and Labrador in 2016 (Lawson & Gosselin 2018).
Taken together, these estimates suggest a stock size of over 35,000 humpback whales in the North Atlantic. This should be considered a minimum estimate for the entire North Atlantic since many of its components are negatively biased and some areas that may also host feeding grounds, such as the mid-Atlantic Ridge (Smith &Pike 2009), are not included.
Abundance Estimates for Humpback Whales in NAMMCO Areas
| Area | Estimate | 95% Confidence Interval | Reference |
|---|---|---|---|
| Iceland / Faroes | 9,867 | 4,854 – 20,058 | Pike et al. 2019 |
| East Greenland | 4,223 | 1,845 - 9,666 | Hansen et al. 2018 |
| West Greenland | 993 | 434 - 2,272 | Hansen et al. 2018 |
| Norway (2014-2018) | 10,708 | 4,906 - 23,370 | Leonard and Øien 2020 |
Breeding ground and ocean basin estimates
The Years of the North Atlantic Humpback (YoNAH) project collected genetic samples and fluke photographs from all North Atlantic feeding grounds and from the West Indies breeding ground from 1992–1993 and used mark-recapture methods to estimate the total population of the North Atlantic as 10,600 (95% CI: 9,300–12,100) (Smith et al. 1999).
A similar study was conducted in 2004–2005, yielding an estimate of 12,312 (95% CI: 8,688–15,954) (Bettridge et al. 2015). Smith and Pike (2009) noted that these estimates are substantially lower than the total of the feeding ground estimates for similar periods, which are themselves negatively biased, as explained above. The YoNAH and later mark-recapture estimates may underestimate the total population because no sampling was conducted at the Cape Verde breeding ground or at other as-yet undescribed breeding grounds. However, the Cape Verde breeding population alone has been estimated to host only about 260 whales (Ryan et al. 2014), which is far too low to account for the discrepancy between feeding ground estimates and the ocean basin mark-recapture estimates. It is also possible that some humpback whales may not make the journey to the breeding grounds every year. Whales have, for example, been observed off Iceland throughout the winter months (Magnusdottir et al. 2014, Víkingsson 2004).
Trends in abundance
Humpback whale numbers appear to have grown rapidly in some areas of the North Atlantic. Under ideal conditions, humpback whale populations can grow at an annual rate of about 11% (Heide-Jørgensen et al. 2012). Aerial surveys conducted around Iceland between 1986–2001 indicated a rate of increase of about 12% per year, however that increase seems to have slowed or stopped since 2001 (Pike et al. 2019, 2020b). A similar rate of 9.4% per year has been observed off West Greenland between 1984–2007 (Heide-Jørgensen et al. 2012), however the most recent estimate from 2015 is lower (Hansen et al. 2018). Numbers in the Northeast Atlantic off Norway have increased dramatically, with estimates from recent surveys being more than double that observed in the 1996-2001 series and more than ten times that estimated in 1995 (Øien 2009, Leonard & Øien 2020a,b). Taken together, these estimates suggest a substantial increase in humpback whale numbers in most areas of the North Atlantic in the late 20th and early 21st centuries.
As these are the largest feeding grounds in terms of whale numbers, these increases should result in similar increases at the breeding grounds. However, numbers at the West Indies breeding ground have increased at a rate of about 2% between 1979 and 2005 (Bettridge et al. 2015). Again, this suggests that a substantial proportion of whales from these feeding grounds must be utilising a breeding ground that has not been directly enumerated, or that they may not occupy the breeding grounds every year (Smith & Pike 2009).
Stock Status
Assigning a “status” to a particular stock of animals is a complex and sometimes controversial exercise that requires, among other things, some knowledge of the historical abundance trend of the stock, an assessment of the reliability of that knowledge, and clearly stated management/conservation goals with respect to the stock. There is no universal definition of stock status used by all organisations and countries. It is therefore important to clearly define stock status before an assessment is made.
Worldwide
The humpback whale is a cosmopolitan species, occupying all world oceans on at least a seasonal basis. Globally, humpback whales have recovered more rapidly than most other baleen whales from overhunting by commercial whalers in the 20th century, probably because they were not a favoured species for whalers and therefore less hunted (Smith & Reeves 2011) but also seem to be able to increase their numbers more rapidly than most other species (Heide-Jørgensen et al. 2012). For instance, humpback whales in the Southern Ocean might even reproduce annually nowadays (likely in response to favourable foraging conditions) , further supporting population recovery (Pallin et al. 2018).
The International Union for Conservation of Nature and Natural Resources (IUCN) uses specific criteria to assign species to one of nine classifications ranging from “Least Concern” to “Vulnerable” to “Extinct” . Under this system, humpback whales were classified globally as being of “Least Concern” for conservation in 2008, primarily because populations are increasing in most areas where data are available and population sizes are probably over 50% of pre-whaling levels in most areas (Reilly et al. 2008). This classification was reiterated in 2018, with a current global population estimated at 135,000 and a mature population at about 84,000, which is higher than the level of three generations ago (Cooke 2018).
North Atlantic
In the North Atlantic, a total catch (including struck and lost) of over 30,000 whales between 1880 and 1940 (Smith & Reeves 2011) apparently reduced the population to such a low level that whaling became unprofitable in most areas. Since 1940, whaling has either ceased or been reduced to very low levels and is now carried out under management regimes that work to ensure the sustainability of the catch. These management regimes seem to contribute towards population recovery in many areas, including around Iceland (Paxton et al. 2009, Pike et al. 2009, 2019, 2020b), off West Greenland (Heide-Jørgensen et al. 2011), off Norway (Leonard & Øien 2020a,b), the Gulf of Maine, and on the West Indies breeding ground (Bettridge et al. 2015).
While humpback whales are recovering or have recovered in most areas, numbers are still low around the Cape Verde Islands, a breeding area where several thousand whales were harvested historically. A small proportion of the whales that use the Norwegian and Icelandic feeding grounds apparently breed in this area (Wenzel et al. 2009). Humpback whales in this area have been estimated to number in the low hundreds (Ryan et al. 2014), although there are no sufficient data to make conclusions regarding population trends.
IWC
The Scientific Committee of the International Whaling Commission (IWC) has attempted to develop an assessment model for North Atlantic humpback whales (IWC 2002, 2003). The model integrated abundance estimates for the entire North Atlantic (Smith et al. 1999) and for several feeding areas, known trends in abundance, historical harvests from all areas, and known life history parameters such as reproductive and natural mortality rates. Punt et al. (2007) furthered this analysis by exploring alternative estimates of abundance and catch, as well as the possible existence of a third breeding ground.
While these modelling efforts were not successful in providing an acceptable fit to estimated population trajectories on some of the feeding and breeding grounds, they do indicate that the North Atlantic humpback whale population has increased substantially since the cessation of whaling and may be approaching or even exceed the estimated pre-whaling levels of between 20,000–26,000 animals. The most recent data on feeding ground abundance, summing to nearly 35,000 animals (see above), supports this suggestion.
NAMMCO
The Scientific Committee of NAMMCO (2010, 2017) carries out assessment of the West Greenland feeding area to determine whether a harvest can be sustainable.
In 2010, a model incorporated current abundance and catch data, historical harvests and estimates of life history parameters indicated that the stock component has likely recovered to (or nearly to) its historical size and that an annual take of up to 20 whales per year would be sustainable (NAMMCO 2010).
The best recent estimates of the size of this stock component is 3,272 (95% CI: 1,300–8,233) in 2007 (Heide-Jørgensen et al. 2012) and 993 (95% CI: 434–2,272) in 2015 (Hansen et al. 2018). Numbers increased at 9.4% per year between 1984–2007 (Heide-Jørgensen et al. 2012) but show a sharp decline by 2015. The reasons for this are not known but may be due to changes in distribution to adjacent areas, such as East Greenland and Canada (Hansen et al. 2018).
Management
Humpback whales continue to be hunted in very small numbers under aboriginal subsistence whaling permits in two areas of the North Atlantic, off West Greenland and St. Vincent and the Grenadines. They are protected in East Greenland and by the Faroe Islands, Iceland and Norway.
A list of laws and regulation in NAMMCO countries regarding hunting of marine mammals (a.o. protection and hunting methods) can be found here.
The humpback whale falls under the international management jurisdiction of the International Whaling Commission (IWC) and NAMMCO.
NAMMCO
NAMMCO provides scientific advice on stock status, sustainable takes, and proposals for conservation and management to its member countries. However, for humpback whale, Greenland, the only NAMMCO member country hunting the species, takes advice from the International Whaling Commission (see below). NAMMCO has however conducted assessment for humpback whales in West Greenland and provided advice on quota in 2010 and 2018.
In 2010, NAMMCO concluded that takes of up to 20 per year would be sustainable (NAMMCO 2010). In 2017, using the Strike Limit Algorithm (SLA) developed by the IWC, NAMMCO concluded that 25 strikes per year were safe in the period 2019-2024 (NAMMCO 2017).
IWC
The IWC began establishing commercial quotas for humpback whales in the 1970s. In 1955, the IWC proposed a quota of 10 humpback whales per year for West Greenland. This was reduced to 9 in 1984 and 8 in 1985. In 1986, the IWC instituted a temporary moratorium on commercial whaling. However, by this time commercial whaling for humpbacks in the North Atlantic had already largely ceased already. Aboriginal Subsistence Whaling could continue under a special scheme, the Aboriginal Subsistence Whaling management program at the IWC.
The recommended strike limits for aboriginal subsistence whaling are based on scientific advice regarding what is considering a sustainable take, as well as the cultural and nutritional needs of the community. The main objectives of the IWC for Aboriginal Subsistence Whaling are: 1) to ensure the risk of extinction is not seriously increased; 2) to enable harvests in perpetuity appropriate to cultural and nutritional requirements, and; 3) to maintain stocks at their highest net recruitment level, and if below that, to ensure they move towards it. Quotas for allowable takes in Aboriginal Subsistence Whaling are, therefore, decided by an integration of both sustainability criteria and determinations of nutritional and cultural needs and are both conservative and sustainable.
Based on the Aboriginal Subsistence Whaling exemption, Greenland requested in 2007 a quota for humpback whales. The Scientific Committee of the IWC completed an Aboriginal Whaling Management Procedure for humpback whales in 2008 (IWC 2008) and advised that catches of 10 humpback whales per year would be sustainable. In 2010, Greenland implemented a catch quota of 9 humpback whales per year for West Greenland, with the possibility of carrying over up to two unused quota animals from one year to the next (Greenland 2012).
In 2018, the IWC advised that a continuation of the annual strike limit of 10 whales would not harm the stock and would meet the Commission’s conservation objectives (IWC 2018).
Regulated hunting in Greenland
Hunting is regulated and administered by the Ministry of Fisheries, Hunting and Agriculture (Greenland 2012) and supervised by the Fisheries Licence Control Authority. Some of the regulations are general to hunting (Home Rule Act no. 12, 29-10-1999, and later amendments in 2001, 2003 and 2008), animal welfare (Home Rule Act no. 25, 18-12-2003), nature protection (Home Rule Act no. 29, 18-12-2003) and hunting permits (Executive order nr. 20, 27-12-2003). Others specifically address the hunting of large whales, such as executive orders no. 11 and 12 from July 16, 2010. In addition to Greenland Government rules, there may also be additional rules set by the municipality.
Under Greenlandic law, all whale products must be consumed locally and no export is permitted (Greenland 2012).
Hunting permits
Catching humpback whales is limited to full-time hunters who have a valid permit to take whales. All prospective hunters must take a course on the handling of the harpoon cannon and whale grenades. Regulations limit the hunt to vessels that are at least 36 feet in length and equipped with a harpoon cannon. The harpoon cannon must be inspected biennially. Locally, wildlife officers monitor whale hunts and ensure that all requirements are met. Once a whale is caught, the hunter is responsible for providing a catch report to municipal authorities, including the time and location of the hunt, biological data such as length, sex, reproductive state of females, stomach contents, and weight of meat products, as well as information on the hunt including the number of grenades used and the estimated time to death (Greenland 2012).
Hunting and Utilisation
Unlike the much faster fin and blue whales, the relatively slow/moving humpback whale was vulnerable to whalers using only sailing ships and rowboats and therefore has a longer history of exploitation in the North Atlantic. Greenland Inuit have taken humpback whales for at least a thousand years, hunting them from kayaks and umiaq (larger skin boats) using hand-held harpoons, floats and lances (Greenland 2012). Early Basque whalers may also have occasionally taken humpback whales, although they specialised in right, bowhead and possibly grey whales.
As with other large whales, humpbacks were hunted primarily for their oil, which was used for lighting, as a lubricant, and later as a food source. The flexible baleen was also used for many purposes for which plastic might be used today, for example for buggy whips, corset stays, and collar stiffeners. If processed on shore, the offal and bones were sometimes used to make fertiliser. Meat for human consumption was not an important product for early commercial whalers but became more important in the 20th century.
Historically
Smith and Reeves (2011) provide a detailed history of humpback whaling in the North Atlantic. Historically, humpback whaling can be divided into roughly two types of fisheries: non-mechanised and mechanised.
Non-mechanism whaling
Non-mechanised whaling used sailing ships and rowboats. The whales were struck with “cold” (non-explosive) harpoons and killed with lances. This type of whaling could be either shore-based, where the whales were taken close to the coast and towed to a station for processing, or pelagic, in which the whale was processed aboard the ship at sea. About 14,000 humpbacks were landed by non-mechanised whaling between 1600–1900, with most being taken in the last hundred years of the period and on the breeding grounds in the West Indies and Cape Verde Islands. So-called ‘Struck and lost rates’ were relatively high in this type of whaling and have been estimated by Smith and Reeves (2011) as 41% of landed catch.
Mechanised whaling – Industrialisation
Mechanised whaling, using faster motorised ships and gun-fired harpoons equipped with explosive grenades, was developed by Norwegian Svend Foyn in the 1870s.
Mechanised whaling, drastically improved the efficiency and safety of the whaling, and made catching the faster-swimming fin and blue whales feasible. Often led by Norwegian companies, this type of whaling rapidly spread throughout the North Atlantic, including Northern Norway, Svalbard and Bear Island, Iceland, the Faroe Islands, West Greenland, Canada, and the humpback breeding grounds at Cape Verde and the West Indies.
The catch of humpbacks in mechanised whaling operations peaked around 1900, with a total of about 7,700 whales landed. The largest catches were taken in Northern Norway, Iceland and Newfoundland. Loss rates were much lower in mechanised whaling than in non-mechanised whaling, as the whale was killed outright or severely injured by the grenade at the same time as it was secured by the harpoon. Smith and Reeves (2011) estimate that the loss rate in mechanised whaling was less than 2% of landed catch.
Whaling for humpbacks ended in most areas by around 1930. At this time, most stocks were reduced to such low levels that whaling was no longer profitable. Furthermore, petroleum products had replaced whale oil for most non-food uses. In total, 21,476 humpback whales are known to have been landed in the North Atlantic, corresponding to a total removal of 30,852 when corrected for struck and lost animals (Smith & Reeves 2011). Catches peaked in the period 1870–1910, corresponding to the advent of mechanised whaling operations in several areas. Some humpback whaling continued in West Greenland and in the Caribbean after 1930, but North Atlantic catches as a whole have never approached the historical levels.
In Iceland, whaling commenced in 1948 with the operations of the Icelandic-owned whaling station at Hvalfjørður. Fin whales were the most significant species, but sei whales and sperm whales were also hunted, and at the beginning also humpback and blue whales but in lesser numbers. Hunting of humpback whales was, however, completely prohibited in 1955.
Commercial whaling of humpback whales was first regulated in 1946, by the International Convention for the Regulation of Whaling. In 1966, the International Whaling Commission prohibited commercial whaling of humpbacks.
Humpback whales are still hunted in very small numbers in Greenland and St. Vincent and the Grenadines under aboriginal subsistence whaling permits and conservative and sustainable quotas set by the IWC.
Whaling in Bequia
A very small hunt for humpback whales continues in Bequia–a small island of the Caribbean nation of St. Vincent and the Grenadines, 0–4 whales per year. The area is in the West Indies breeding ground, so whales taken here could be from any of the North Atlantic feeding grounds. The IWC established a block quota of 24 for the period 2013–2018 under the Aboriginal Subsistence Whaling management program (IWC 2015). In 2018, the IWC advised that on the basis of the information available and the size of the requested catch (an average of four annually), continuation of the present limits would not harm the stock (IWC 2018).
The whales are hunted using a rowing boat and a hand-thrown harpoon with the line attached to the boat. The whale is killed using a shoulder gun firing an explosive grenade or hand lances (Adams 1971). The whale is then towed to shore for processing. Today motorboats are sometimes used to assist in this process. The whale is processed on shore and the meat and oil are distributed, bartered or sold on Bequia or neighbouring islands within St Vincent and the Grenadines. No export is permitted.
Whaling in Greenland
Whaling in West Greenland has a very long history. The Thule Inuit (who occupied Greenland beginning around the year 1000) were skilled marine hunters who hunted several species of cetaceans, including the humpback whale. They used the kajaq (kayak) and the larger umiaq skin boats to pursue whales on the open seas. Whales were struck with hand-thrown, detachable head harpoons, with lines attached to skin floats. Hunters often wore the atallaaq, a drysuit made of waterproof seal skin, in which they would actually jump onto the whale to deliver the lethal lance strikes (Greenland 2012). Contact with American and European bowhead whalers began in the 18th century and Greenlandic Inuit were quick to adopt some of their equipment and techniques, including faster wooden boats, metal-edged harpoons, lances, flensing tools, and hemp ropes.
Humpback whaling has therefore a long history in West Greenland and has been conducted since the 1700s and possibly even before (Greenland 2012, Smith & Reeves 2011).
The 20th century
By the beginning of the 20th century, commercial whalers had reduced populations of bowhead and humpback whales in Greenlandic waters to the point that whaling from small boats was no longer feasible. As a result, most hunting for large whales in the middle part of the century was carried out by one vessel operated by the Royal Greenland Trading Company–the Sonja. This vessel operated from 1924–1958, with a brief cessation during the Second World War.
The Sonja was a steam-powered vessel that used mechanised whaling methods, including a harpoon cannon and explosive grenades. Whales were towed to villages on the Greenland west coast, where they were flensed by local people in return for a portion of the catch. This provided a welcome and much needed source of food for the people of Greenland. Most of the catch consisted of fin and blue whales, but some humpback whales were also taken (Caulfield 1997).
Beginning in the 1940s, some Greenlanders began to equip their fishing vessels with harpoon cannons to participate in seasonal whaling. Minke whales were the main target of this hunt, but some fin, blue and humpback whales were also taken (Caulfield 1997, Greenland 2012). These vessels generally distributed their catch locally or sold it to neighbouring villages or larger centres.
Introduction of the quota
In 1955, the IWC proposed a quota of 10 humpback whales per year for West Greenland. This was reduced to 9 in 1984 and 8 in 1985. Whaling for humpbacks was prohibited in 1986 as there was concern that the stock had been depleted. In 2007, Greenland requested a quota for humpback whales under the Aboriginal Subsistence Whaling management program at the IWC. The Scientific Committee of the IWC completed an Aboriginal Whaling Management Procedure for humpback whales in 2008 (IWC 2008) and used it to advise that catches of 10 humpback whales per year would be sustainable.
In 2010, Greenland implemented a catch quota of 9 humpback whales per year for West Greenland, with the possibility of carrying over up to two unused quota animals from one year to the next (Greenland 2012).
Modern whaling in Greenland
Whaling for humpback whales resumed in West Greenland in 2010 after a cessation of 30 years However, during this period, Greenlandic whalers continued to take minke and fin whales, so the skills of whaling were not lost. Whaling for large whales in Greenland is done using fishing vessels equipped with harpoon cannons and other necessary equipment. Only the largest vessels (over 36 feet in length) can participate in the hunt for humpback whales.
The primary weapon used is the 50 mm Kongsberg harpoon cannon. This fires a harpoon with a rope attached into the whale. As the harpoon enters the whale, a hook triggers the firing of a “Whale Grenade 99” loaded with 30–45 g of penthrite explosive into the whale. A timed fuse detonates the grenade, killing or severely injuring the whale through concussion and tissue damage. Gunners generally target the heart and lung area by aiming for the area close to the pectoral fins (NAMMCO 2010, 2015).
Since the resumption of humpback whaling in 2010, median Time to Death (TTD) for humpback whales in the Greenlandic hunt has ranged from 7 minutes in 2010 to 15 minutes in 2013. The percentage of whales that die instantaneously after being hit has ranged from 17% in 2010, 2013 and 2014 to 50% in 2011. These values are similar to those for fin whales, which are hunted using similar methods (NAMMCO 2015). Struck and lost is generally low, ranging from 0% in most years to a high of 43% in 2012. In recent years, NAMMCO has held two Expert Group meetings on reducing TTD in whale hunts (NAMMCO 2010, 2015). In addition, NAMMCO has developed a Hunting Manual for baleen whales, available free of charge online in English, Norwegian and Greenlandic (NAMMCO 2014).
Use of the meat
Once the whale is secured, it is towed to shore to a suitable butchering area at the next high tide and the whale is flensed at low tide. The meat is shared amongst the participants in the hunt, or sold at local markets, particularly the open-air markets (Greenlandic Kalaalimineerniarfik, Danish brædtet) but also to supermarkets, hotels and restaurants. Operating a whaling vessel equipped with a harpoon cannon can be very expensive. The cannon alone might cost US $60,000 and each grenade costs as much as US $1,500 (Greenland 2012), and fuel is also very expensive in Greenland. Therefore, whalers require some monetary return in order to continue whaling. No export of whale products from Greenland is permitted though.
Catches in NAMMCO member countries since 1992
| Country | Species (common name) | Species (scientific name) | Year or Season | Area or Stock | Catch Total | Quota (if applicable) |
|---|---|---|---|---|---|---|
| Greenland | Humpback whale | Megaptera novaeangliae | 2023 | West | 2 | 10 |
| Greenland | Humpback whale | Megaptera novaeangliae | 2022 | West | 2 | 10 |
| Greenland | Humpback whale | Megaptera novaeangliae | 2021 | West | 6 | 10 |
| Greenland | Humpback whale | Megaptera novaeangliae | 2020 | West | 1 | 10 |
| Greenland | Humpback whale | Megaptera novaeangliae | 2019 | West | 4 | 10 |
| Greenland | Humpback whale | Megaptera novaeangliae | 2018 | West | 6 | 12 |
| Greenland | Humpback whale | Megaptera novaeangliae | 2017 | West | 2 | 12 |
| Greenland | Humpback whale | Megaptera novaeangliae | 2016 | West | 5 | 12 |
| Greenland | Humpback whale | Megaptera novaeangliae | 2015 | West | 6 | 12 |
| Greenland | Humpback whale | Megaptera novaeangliae | 2014 | West | 7 | 12 |
| Greenland | Humpback whale | Megaptera novaeangliae | 2013 | West | 8 | 10 |
| Greenland | Humpback whale | Megaptera novaeangliae | 2012 | West | 10 | 10 |
| Greenland | Humpback whale | Megaptera novaeangliae | 2011 | West | 8 | 9 |
| Greenland | Humpback whale | Megaptera novaeangliae | 2010 | West | 9 | 9 |
| Greenland | Humpback whale | Megaptera novaeangliae | 1992-2009 | West | *No reported catches | |
This database of reported catches is searchable, meaning you can filter the information, e.g. by country, species or area. It is also possible to sort it by the different columns, in ascending or descending order, by clicking the column you want to sort by and the associated arrows for the order. By default, 30 entries are shown, but this can be changed in the drop-down menu, where you can decide to show up to 100 entries per page.
Carry-over from previous years are included in the quota numbers, where applicable.
You can find the full catch database with all species here.
You can find a complete file with all comments and explanations here, under Overview Documents.
For any questions regarding the catch database, please contact the Secretariat at nammco-sec@nammco.no.
Other Human Impacts
Whale watching
Humpback whales are prime targets for whale watching because they have a coastal distribution accessible to shore-based operators, exhibit splendid surface behaviour, and are relatively easy to approach. Whale watching has experienced spectacular growth worldwide in the past few decades, with commercial operations springing up in areas where whales can be accessed within a reasonable distance from the coast. The whale watching industry was estimated to have a worldwide value of over 2.5 billion US dollars in 2009 (O’Connor et al. 2009).
Commercial whale watching operations that specialise in humpback whales operate in Norway, Iceland, West Greenland and other North Atlantic nations. In Iceland, the whale watching industry has grown explosively in recent years and had a value of nearly 17 million US dollars annually in 2008 and over 120,000 participants (O’Connor et al. 2009, Martin 2012). Other benefits include employment, public education and assistance in research and monitoring programs.
Impacts
While usually considered a relatively benign and sustainable “use” of marine mammals, whale watching can have short-term impacts on individuals or groups of whales which, if experienced frequently, can lead to long lasting negative effects on a population level. Humpback whales may react to vessel noise and/or the simple proximity of vessels in a number of ways. The most frequently observed reaction is an increase in swimming speed when boats are nearby (Boye et al. 2010, Bettridge et al. 2015). There may also be an increase in surface behaviours and a tendency to move away from vessels, although the opposite effect is sometimes observed (Magnúsdóttir 2011, Martin 2012, Bettridge et al. 2015).
The long-term effects of high-intensity whale watching on humpback whale populations and other cetacean species are not well known, but could include cumulative impacts of noise, reduced fitness through increased energy spent avoiding vessels or diving for longer, and changes to their normal habitat (Lusseau & Bejder 2007, Schuler et al. 2019)(Weinrich and Corbelli (2009) monitored individual humpback whales exposed to different levels of whale watching activity and found no detectable effects on calf production or survival. However, such effects are notoriously difficult to detect and may require studies lasting at least the generation time of the species.
Mitigation
The potential negative effects of whale watching can be mitigated with suitable regulation or voluntary codes of conduct among whale watching operations. Such regulations generally includes speed limits when operating in whale-rich waters, a close approach distance limit, and a requirement to stop if the vessel is approached by whales (Martin 2012). To date, NAMMCO member countries have used industry codes of conduct to regulate whale watching. The efficacy of or compliance with these codes has not been assessed.
Conflict with subsistence hunting
Whale watching specialising in humpback whales has become popular in the Nuuk fjord system of West Greenland. Most of the whales that use this area do so for a few weeks each summer and return year after year. This leads to a potential conflict between whale hunting and whale watching, as the removal of the few “resident” whales could have an adverse effect on the local whale watching industry (Boye et al. 2010).
By-catch and entanglement
Among large whales, humpbacks seem particularly prone to becoming entangled in fishing gear. They consume pelagic fish that are also sought by fishermen and are often found in coastal areas with intense fishing activity. A large proportion of some stocks may become entangled at some point in their lives. In the Gulf of Maine, between 48–65% of humpback whales have scars that likely resulted from gear entanglement (IWC 2002). Yearling whales are more prone to entanglement than older ones, but whales of all ages can become entangled.
While many whales clearly survive entanglement events, some do not, and a large proportion of dead-stranded humpback whales show evidence of entanglement in some areas (Henry et al. 2014). Mark-recapture studies have shown that juveniles are less likely than adults to survive gear entanglements and that death due to entanglement may account for 3–4% of total mortality in the Gulf of Maine (Bettridge et al. 2015). Females that showed signs of entanglement injury produced fewer calves than those that did not (IWC 2002), so there may be population-level effects in addition to direct mortality.

© NOAA
Gear type
Humpback whales are particularly vulnerable to static gear (such as gill nets and lobster and crab pots), entangling in the buoy or groundlines (Johnson et al. 2005). The rate of entanglement off Newfoundland, for example, dropped from 64 per year to 19 per year after the moratorium on the inshore cod fishery was introduced in 1992 (IWC 2013). In some areas, rescue and release programs have been successful in freeing a high percentage of entangled whales (IWC 2002). However, many entangled whales are presumably unobserved. Changes in gear type, such as reducing the use of static gear in areas of high whale density, or including “weak links” in buoy and float lines, may also be effective in reducing death or injury due to entanglement.
Large whale entanglement response
Challenges with entanglements of humpback and killer whales in the herring fisheries in Northern Norway have arisen after these whales started coming into the fjords in 2011. As a response to this, the Norwegian Coast Guard and the Norwegian Directorate of Fisheries’ Sea Surveillance Service have been given special training in large whale entanglement response (National Progress Report Norway 2018).
Vessel strikes
Humpback whales are second only to fin whales in the frequency of reported vessel strikes (Bettridge et al. 2015). Ship strikes may be the cause of death in 30% or more of dead stranded humpback whales on the US eastern seaboard (Henry et al. 2014, Laist et al. 2001). Most lethal ship strikes are by ships greater than 80 m in length and travelling at a speed in excess of 14 knots (Laist et al. 2001). Experimental studies have shown that strike rates drop by over 90% if ship speed is reduced below 12.5 knots (IWC 2016). An obvious mitigation measure is therefore to reduce ship speed in areas of high whale density, as well as employing an observer when traveling in whale waters.
Climate change
Whales may respond to a changing climate in at least three different ways: by redistribution (i.e., changing their seasonal migratory pattern), by adaptation to the change, or by extinction. Humpback whales as a species have shown large historical changes in distribution in the North Atlantic, so it seems likely that they will continue to respond to a changing environment through redistribution, to the extent that this is possible for them (Bettridge et al. 2015). A warming climate may in fact open up more of the ice-free boreal habitat favoured by humpback whales as a feeding ground than is presently available to them.
There is already evidence that climate-induced changes in humpback whale behaviour are occurring. Higdon and Ferguson (2011) found that humpback whales were being sighted in areas of the Canadian Eastern Arctic where they had never been seen before, and postulated that a reduction in summer ice cover in Hudson Strait had allowed the whales to penetrate into the area. Ramp et al. (2015) found that humpback whales were arriving at the Gulf of St. Lawrence feeding ground about four weeks earlier in 2010 than they did in 1987, corresponding to a change in arrival date of about one day per year. They were also departing earlier by a similar length of time. This change was most closely correlated to a rise in sea surface temperature over the period, which also corresponded to an earlier ice-free date.
While the effects of climate change on humpback whales cannot be predicted, it is likely that continued warming and a reduction in Arctic ice cover will result in a northward shift in summer distribution in some areas, and possible changes in seasonal occupation in others. Climate change is likely less of a danger to the adaptable humpback whale than to some Arctic, ice dependent species, for which the total amount of habitat is being reduced.
Acoustic pollution
Humpback whales produce a wide variety of sounds that they use for communication, foraging and reproduction. Noise created from shipping, seismic exploration, and military or other sonars can affect humpback whales by masking their sounds and reducing their effective range. The ocean is rapidly becoming a more noisy environment and anthropogenic sound production in coastal areas is thought to have doubled each decade for the last 30 years (Bettridge et al. 2015).
Humpback whales respond to increased background noise by changing their foraging behaviour (for instance descending more slowly and performing fewer feeding attempts at depth), higher respiration rate and swim speed, as well as altered calling behaviour (calling louder in increasing noise, but also calling less frequently the louder it gets) (Blair et al. 2016; Fournet et al. 2018; Sprogis et al. 2020).
Acoustic interference with humpback whales can be mitigated by reducing or eliminating seismic and sonar activities in areas and seasons when whales are present in high densities, especially during the mating season when singing whales are present. Additionally, lowering speed limits and synchronizing ship traffic may lower the negative impacts of noise on humpback whales (Frankel & Gabriele 2017).
Contaminants
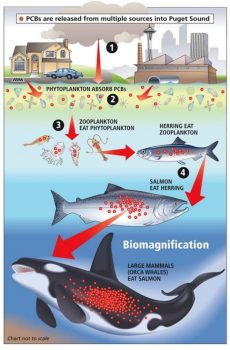
Biomagnification through the example of persistent organic pollutants (POPs). Pollutants enter the aquatic environment and are passing through several trophic levels, accumulating over time and peaking in animals at high trophic levels like killer whales. Source: https://cimioutdoored.org/bioaccumulation-and-biomagnification-increasingly-concentrated-problems/
As long-lived top level marine predators, humpback whales tend to accumulate organic pollutants such as pesticides in their blubber through a process called biomagnification. Biomagnification describes a process by which pollutants are absorbed from the water by organisms at the bottom of the food chain (such as phytoplankton), which then serve as food for other organisms higher in the food web like zooplankton that feed fish, which are finally consumed by marine mammals. Through this chain, pollutants accumulate in each organism and may lead to fatal levels in apex predators like fish and marine mammals. These pollutants can also be transferred from mother to calf through lactation (Bettridge et al. 2015). Known consequences of such biomagnification of organic contaminants in marine mammals include impaired immunity and increased susceptibility to disease, neurotoxicity, and reproductive decline (Elfes et al. 2010). However, concentrations of contaminants in humpback whale tissue are generally below those known to cause such impairments in other animals (Ryan et al. 2013). Concentrations vary greatly between whales sampled in different areas, which probably reflects the extent of contamination present in humpback whale prey. For example, concentrations of organic contaminants were much lower in whales sampled around the Cape Verde Islands than in those sampled in the Gulf of St Lawrence or Gulf of Maine, perhaps reflecting the levels of agricultural runoff in the latter areas (Ryan et al. 2013).
Some types of algal blooms produce toxins harmful to marine mammals and these blooms have been associated with inflow of untreated industrial and domestic wastewater. Toxins associated with an algal bloom killed at least 14 humpback whales in 1987–1988 off the US eastern seaboard and such toxins are often found in the tissues of dead stranded whales (Bettridge et al. 2015). However, the population level effects of these toxins are not known.
Research in NAMMCO Member Countries
All NAMMCO member countries have participated in the North Atlantic Sightings Surveys and the humpback whale has been a target species in all areas. These surveys are coordinated through the Scientific Committee of NAMMCO. In addition, each country conducts other important research on the biology and ecology of humpback whales.
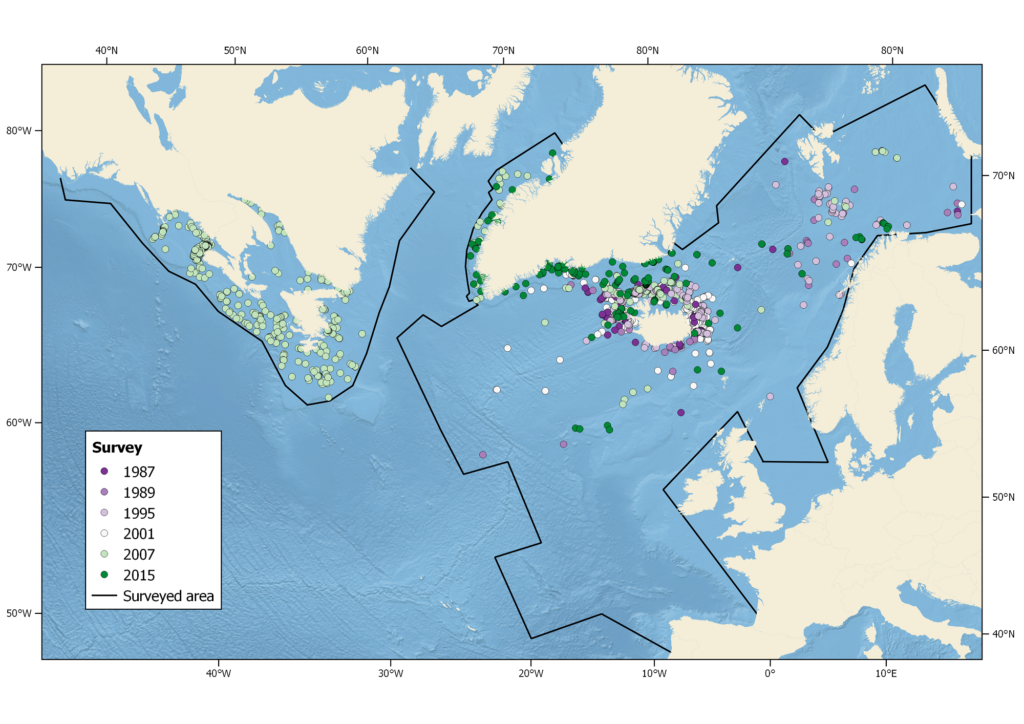
Summer sightings of humpback whales during all NASS from 1987-2015. Black lines indicate areas surveyed (not all areas surveyed in each year).
Greenland
As one of only two countries in the North Atlantic that continue to hunt humpback whales, Greenland has participated in the NASS surveys and used aerial surveys to cover the west coast of Greenland from Cape Farewell north to about 70°N. In 2015, the east coast of Greenland was also covered, which revealed relatively large numbers of humpback whales in that area. This may mean that these waters constitute a feeding area for humpback whales, at least in some years.
Greenland has also contributed fluke photographs and genetic samples to international humpback whale databases and participated in the Years of the North Atlantic Humpback (YoNAH) and follow-up projects. The collection of fluke and dorsal fin identification photographs from West Greenland taken by the public is ongoing (National Progress Report Greenland 2019).
Satellite tagging
Greenland has been very active in tracking whales using satellite tags. This has led to a greater understanding of the local and migratory movements of humpback whales (Heide-Jørgensen & Laidre 2007). Tagging efforts have also advanced our knowledge of how lunge feeding by humpback whales occurs (Simon et al. 2012).
In 2019, fieldwork on humpback whales in Nuuk included photo-identification, biopsy sampling, satellite telemetry and drone recordings (National Progress Report Greenland 2019). In 2019, humpback whales were a target species for telemetry studies in both West and East Greenland.
Hunting methods
As a whaling nation, Greenland monitors its whale hunts and participates in efforts to improve hunting methods that reduce time-to-death (TTD) and struck and lost rates (NAMMCO 2010, 2015). Since the resumption of humpback whaling in 2010, median TTD for humpback whales in the Greenlandic hunt has ranged from 7 minutes in 2010 to 15 minutes in 2013. The percentage of whales that die instantaneously after being hit has ranged from 17% in 2010, 2013 and 2014 to 50% in 2011. These values are similar to those for fin whales which are hunted using similar methods (NAMMCO 2015).
Whale-watching
Whale-watching has become a valuable part of the tourist industry in Greenland, particularly in the fjord system surrounding the capital city, Nuuk. Research in this area has focussed on quantifying the effect of close approaches of whale watching vessels on humpback whale behaviour. The most frequently observed reaction is an increase in swimming speed when boats are nearby (Boye et al. 2010). The humpback whales that use this area tend to return year after year, leading to the suggestion that hunters should avoid taking these whales to support the whale watching industry.
Iceland
The Marine and Freshwater Research Institute (MRI) is the Icelandic governmental body responsible for research concerning conservation and management of cetaceans in Icelandic waters. The MRI’s main research activities on humpback whales in recent decades have focused on the following topics.
1) Distribution and abundance.
This has primarily occurred through participation in large scale international surveys but also using data from opportunistic platforms such as fish- or oceanographic research cruises, and commercial whaling and whale watching platforms. In 2019, humpback whales were the primary species of a whale observation effort during ecosystem surveys focused on capelin (National Progress Report Iceland 2019).
2) Photo-identification studies.
The MRI participated in the YoNAH project in the early 1990s and has continued photo-id studies ever since, including the maintenance of the Icelandic central photo-identification database (see below). In 2019, the catalogue had records for over 1050 unique individuals in Icelandic water (National Progress Report Iceland 2019).
3) Genetics.
Samples obtained from biopsies (including YoNAH) as well as from stranded or by-caught animals have been used in international population genetic studies.
4) Satellite telemetry.
Since the late 1990s, the MRI has used satellite telemetry for research on several cetacean species, including humpback whales. In 2019, 10 humpback whales were instrumented with satellite tags in Icelandic and adjacent waters as a part of the program, with an additional nine Limpet tags also deployed (National Progress Report Iceland 2019).
5) Strandings and net entanglements.
The MRI is responsible for monitoring strandings and by-catch of cetaceans in Icelandic waters and there is research based on both monitoring and mitigation efforts. In 2019, research on entanglement scar analysis estimated that 25% of the Icelandic humpback whale population had been entangled in fishing gear at least once (at a rate of 2% of the population per year) and that the use of acoustic deterrents on fishing gear could prevent damage caused by humpback whales incidentally becoming encircled in seine nets (National Progress Report Iceland 2019).
Iceland has developed a Whale Photo Identification database that allows cataloguing of photographs and photo-matching to identify re-sightings. While several species are included, the humpback whale has been the main focus of photo-ID work. To date, the catalogue documents 1050 unique individuals in Icelandic waters. Several of these individuals have been sighted two or more times, providing information about site fidelity, migration, stock identity and life history. In addition to the photo collection, biopsy samples for genetic and other analyses have been collected from more than 70 humpback whales in Icelandic waters.
Additional Research Topics
Research in Iceland has also investigated the singing behaviour (a breeding display) of male humpback whales in sub-arctic Icelandic waters during the winter. The findings showed continual singing from January to March, with song progressions similar to those found on breeding grounds (National Progress Report Iceland 2019).
A current collaborative research project on the life-history strategy is investigating the body condition and physical and acoustic behaviour of humpback whales in the subarctic waters of Iceland during the winter in comparison to other seasons. In 2019, a total of 40 biopsies (skin/blubber) were collected in four cruises in Icelandic coastal waters for this research. The information collected will be used in a variety of studies, including research on feeding ecology (stable isotopes/fatty acids), stock structure (DNA) and seasonality in reproduction (hormones). The project also involves tagging, photo-identification and behavioural observations.
Norway
Norway surveys a huge area of the Barents, Norwegian and North Seas on a six-year rotation, covering a part of the area each year. While minke whales are the primary target species of the survey, all species including humpback whales are counted. Norway also contributes fluke photographs and genetic samples to international humpback whale databases and participated in the YoNAH and follow-up projects.
Ecological relationship
A major focus of Norwegian research has been to understand the ecological relationships between baleen whales, pelagic fish (such as capelin and Arctic cod) and their invertebrate prey (krill and pelagic amphipods). A series of surveys conducted jointly with Russia in the Barents Sea south of Svalbard between 2003 and 2007 collected simultaneous data on the abundance of krill, amphipods, pelagic fish and cetaceans (Skern-Mauritzen 2011). Baleen whales including humpbacks were found at highest density in a narrow arc along the north edge of the polar front, which corresponded closely to the highest densities of krill in the area. While this suggests that humpback whales graze primarily on krill in this area, the abundance of capelin was at a low level during most of this period. Future work will address how the distribution of cetaceans responds as ecosystem conditions change.
Satellite tagging & foraging behaviour
The “weShare” project coordinated by researchers at Norwegian Institute of Marine Research has used advanced electronic accelerometer/camera tags attached with suction cups to ~25 humpback whales that visited Northern Norway between 2013-2017. This was done to study their foraging behaviour in more detail, estimate prey consumption rates, and estimate body condition. The institute has also developed a novel approach for detecting feeding events of humpback whales from both high-resolution 3D acceleration data and from 2D time-depth dive data (National Progress Report Norway 2019).
Mapping behaviour and migrations
The Whaletrack project was initiated in 2013 with an aim to map the humpback and killer whale behaviour and migrations related to their winter aggregations in the Northern Norwegian fjords. In 2018, the Whalefeast project was also included under the Whaletrack framework. The project is lead by UiT the Arctic University of Norway, which works in close cooperation with the Institute for Marine Research (IMR, Tromsø and Bergen). The project also includes close cooperation with other Norwegian and international institutions and includes several PhD and MSc candidates.
The main purpose of the Whaletrack project is to develop better knowledge of the behaviour of humpback and killer whales before, during and after the period they feed on overwintering herring in the fjords or off the coast of Northern Norway. Whilst the project has focused on mapping the horizontal and vertical migration patterns of humpback and killer whales, the new Whalefeast project (2018–2021) will also include closer cooperation with the fisheries and tourism industry, as well as using eDNA-techniques in addition to already collected data. It will include social science studies of the impacts that the whale arrivals have and have had on the tourist and fisheries industries (National Progress Report Norway 2018).
Photo-identification studies
Identification photos of humpback whales are also collected in Norway, most of which are submitted through the North Norwegian Humpback Whale Catalogue. Since 2010, more than 900 individual humpback whales have been identified in Northern Norway, matching with whales observed in Iceland, Ireland, the Cape Verde Islands and the Carribbean (National Progress Report Norway 2018).
The weShare project (described above) has also carried out a pilot study to evaluate the use of aerial drones to estimate whale abundance and supported the collection of photo IDs of humpback whales (National Progress Report Norway 2019).
Adams, J.E. (1971). Historical Geography of Whaling in Bequia Island, West Indies. Caribbean Studies, 11, 55-74.
Bettridge, S., Baker, C.S., Barlow, J., Clapham, P.J., Ford, M., Gouveia, D., Mattila, D.K., Pace, R.M., III, Rosel, P.E., Silber, G.K. and Wade, P.R. (2015). Status review of the humpback whale (Megaptera novaeangliae) under the Endangered Species Act. U.S. Department of Commerce, NOAA Technical Memorandum NMFS NOAA-TM-NMFS-SWFSC-540. National Oceanic and Atmospheric Administration, NOAA Fisheries, Southwest Fisheries Science Center, Miami, FL. Available at http://www.fisheries.noaa.gov/pr/species/Status%20Reviews/humpback_whale_sr_2015.pdf
Blair, H. B., Merchant, N. D., Friedlaender, A. S., Wiley, D. N., Parks, S. E. (2016). Evidence for ship noise impacts on humpback whale foraging behaviour. Biology Letters, 12, 8. https://doi.org/10.1098/rsbl.2016.0005
Boye, T.K., Simon, M. and Madsen, P.T. (2010). Habitat use of humpback whales in Godthaabsfjord, West Greenland, with implications for commercial exploitation. Journal of the Marine Biological Association of the United Kingdom, 90, 1529-1538. https://doi.org/10.1017/S0025315410000755
Caulfield, R.A. (1997). Greenlanders, whales and whaling: Sustainability and self-determination in the Arctic. University Press of New England, Hanover, NH.
Clapham, P. J. (2018). Humpback Whale – Megaptera novaeangliae. Encyclopedia of Marine Mammals (Third Edition), 489-492.
Clapham, P., Barlow, J., Bessinger, M., Cole, T., Mattila, D., Pace, R., Palka, D., Robbins, J. and Seton, R. (2003). Abundance and demographic parameters of humpback whales from the Gulf of Maine, and stock definition relative to the Scotian Shelf. Journal of Cetacean Research and Management, 5, 13-22. Available at https://archive.iwc.int/pages/search.php?search=%21collection15&k=
Clapham, P. J. & Mead, J.G. (1999). Megaptera novaeangliae. Mamm. Spec. 604, 1–9.
Cooke, J.G. 2018. Megaptera novaeangliae. The IUCN Red List of Threatened Species 2018: e.T13006A50362794. https://dx.doi.org/10.2305/IUCN.UK.2018-2.RLTS.T13006A50362794.en
Corkeron, P.J. and Connor, R.C. (1999). Why do baleen whales migrate? Marine Mammal Science, 15, 1228–1245. https://doi.org/10.1111/j.1748-7692.1999.tb00887.x
Derville, S., Torres, L. G., Zerbini, A. N., Oremus, M., Garrigue, C. (2020). Horizontal and vertical movements of humpback whales inform the use of critical pelagic habitats in the western South Pacific. Scientific Reports, 10. https://doi.org/10.1038/s41598-020-61771-z
Dunlop, R. A., Cato, D. H. & Noad, M. J. (2008) Non-song acoustic communication in migrating humpback whales (Megaptera novaeangliae). Mammal Sci.24, 613–629. https://doi.org/10.1111/j.1748-7692.2008.00208.x
D’Vincent, C. G., Nilson, R. N. & Hanna, R. E. (1985).Vocalization and coordinated feeding behavior of the humpback whale in Southeastern Alaska. Reports Whales Res. Inst.36, 41–47.
Elfes, C.T., VanBlaricom, G.R., Boyd, D., Calambokidis, J., Clapham, P.J., Pearce, R.W., … and Krahn, M.M. (2010). Geographic variation of persistent organic pollutant levels in humpback whale (Megaptera novaeangliae) feeding areas of the North Pacific and North Atlantic. Environmental Toxicology and Chemistry, 29, 824-834. https://doi.org/10.1002/etc.110
Ford, J.K.B., and Reeves, R.R. (2008). Fight or flight: Antipredator strategies of baleen whales. Mammal Review, 38, 50-86. https://doi.org/10.1111/j.1365-2907.2008.00118.x
Fournet, M. E. H., Gabriele, C. M., Culp, D. C., Sharpe, F., Mellinger, D. K., Klinck, H. (2018). Some things never change: multi-decadal stability in humpback whale calling repertoire on Southeast Alaskan foraging grounds. Scientific Reports, 8.
Fournet, M. E. H., Matthews, L. P., Gabriele, C. M., Haver, S., Mellinger, D. K., Klinck, H. (2018). Humpback whales Megaptera novaeangliae alter calling behaviour in response to natural sounds and vessel noise. MEPS, 607, 251-268. https://doi.org/10.3354/meps12784
Fournet, M. E. H., Szabo, A. & Mellinger, D. K. Repertoire and classification of non-song calls in Southeast Alaskan humpback whales (Megaptera novaeangliae). Acoust. Soc. Am.137, 1–10 (2015). DOI: 10.1121/1.4904504
Frankel, A. S., Gabriele, C. M. (2017). Predicting the acoustic exposure of humpback whales from cruise and tour vessel noise in Glacier Bay, Alaska, under different management strategies. Endangered Species Research, 34, 397-415. DOI:10.3354/esr00857
Garland, E. C., Goldizen, A. W., Rekdahl, M. L., Constantine, R., Garrigue, C., Hauser, N. D., Poole, M. M., Robbins, J., Noad, M. J. (2011). Dynamic horizontal cultural transmission of humpback whale song at the ocean basin scale. Biol., 21, 678-691. DOI: 10.1016/j.cub.2011.03.019
Garland, E. C., McGregor, P. K. (2020). Cultural Transmission, Evolution, and Revolution in Vocal Displays: Insights From Bird and Whale Song. Psychol., 11. https://doi.org/10.3389/fpsyg.2020.544929
Garland, E. C., Rendell, L., Lilley, M. S., Poole, M. M., Allen, J., Noad, M. J. (2017). The devil is in the detail: quantifying vocal variation in a complex, multi-levelled, and rapidly evolving display. Acoust. Soc. Am., 142, 460-472. https://doi.org/10.1121/1.4991320
Genov, T. 2023. Megaptera novaeangliae (Europe assessment). The IUCN Red List of Threatened Species 2023: e.T13006A219002308. Accessed on 20 December 2023.
Gong, Z., Jain, A.D., Tran, D., Yi, D.H., Wu, F., Zorn, A., … and Makris, N.C. (2014). Ecosystem scale acoustic sensing reveals humpback whale behavior synchronous with herring spawning processes and re-evaluation finds no effect of sonar on humpback song occurrence in the Gulf of Maine in Fall 2006. PloS ONE, 9(10), e104733. https://doi.org/10.1371/journal.pone.0104733
Greenland. (2012). White paper on the management and utilization of large whales in Greenland. IWC/64/ASW/X. Available at https://nammco.no/wp-content/uploads/2020/05/greenland-whitepaper-on-whaling-2018-iwc-final.pd_.pdf
Hansen, R.G., Boye, T.K., Larsen, R.S., Nielsen, N.H., Tervo, O., Nielsen, R.D., Rasmussen, M.H., Sinding, M.H.S. and Heide-Jørgensen, M.P. (2018). Abundance of whales in West and East Greenland in summer 2015. NAMMCO Scientific Publications 11. https://doi.org/10.7557/3.4689
Heide-Jørgensen, M.P. and Laidre, K.L. (2007). Autumn space-use patterns of humpback whales (Megaptera novaeangliae) in West Greenland. Journal of Cetacean Research and Management, 9, 121-126. Available at https://archive.iwc.int/pages/search.php?search=%21collection15&k=
Heide-Jørgensen, M.P., Borchers, D.L., Witting, L., Laidre, K.L., Simon, M.J., Rosing-Asvid, A. and Pike, D.G. (2008). Estimates of large whale abundance in West Greenland waters from an aerial survey in 2005. Journal of Cetacean Research and Management, 10, 119-129. Available at https://archive.iwc.int/pages/search.php?search=%21collection15&k=
Heide-Jørgensen, M.P., Laidre, K.L., Hansen, R.G., Burt, M.L., Simon, M., Borchers, D.L., … and Teilmann, J. (2012). Rate of increase and current abundance of humpback whales in West Greenland. Journal of Cetacean Research and Management, 12, 1-14. Available at https://archive.iwc.int/pages/search.php?search=%21collection15&k=
Heide-Jørgensen, M.P., Simon, M.J. and Laidre, K.L. (2007). Estimates of large whale abundance in Greenland waters from a ship-based survey in 2005. Journal of Cetacean Research and Management, 9, 95-104. Available at https://archive.iwc.int/pages/search.php?search=%21collection15&k=
Henry, A.G., Cole, T.V.N., Hall, L., Ledwell, W., Morin, D. and Reid, A. (2014). Mortality determinations for baleen whale stocks along the Gulf of Mexico, United States east coast, and Atlantic Canadian provinces, 2008 – 2012. US Dept Commer, Northeast Fish Sci Cent Ref Doc. 14-10, 17 p.
Herman, L. M. (2017). The multiple functions of male song within the humpback whale (Megaptera novaeangliae) mating system: review, evaluation and synthesis. Rev., 92, 1795-1818. https://doi.org/10.1111/brv.12309
Higdon, J.W. and Ferguson, S.H. (2011). Reports of humpback and minke whales in the Hudson Bay region, eastern Canadian Arctic. Northeastern Naturalist, 18, 370-377. https://doi.org/10.1656/045.018.0309
International Whaling Commission (IWC). (2002). Report of the Scientific Committee. Journal of Cetacean Research and Management, 4 (suppl.). Available at https://archive.iwc.int
International Whaling Commission (IWC). (2008). Report of the Scientific Committee. Journal of Cetacean Research and Management, 11 (suppl.). Available at https://archive.iwc.int
International Whaling Commission (IWC). (2013). Report of the Scientific Committee. Journal of Cetacean Research and Management, 14 (suppl.). Available at https://archive.iwc.int
International Whaling Commission (IWC). (2015). Report of the Scientific Committee. Journal of Cetacean Research and Management, 16 (suppl.). Available at https://archive.iwc.int
International Whaling Commission (IWC). (2016). Report of the Scientific Committee. Journal of Cetacean Research and Management, 17 (suppl.). Available at https://archive.iwc.int
International Whaling Commission (IWC). (2018). Report of the Scientific Committee. Available at https://archive.iwc.int
Johnson, A., Salvador, G., Kenney, J., Robbins, J., Kraus, S., Landry, S. and Clapham, P. (2005). Fishing gear involved in entanglements of right and humpback whales. Marine Mammal Science, 21, 635-645. https://doi.org/10.1111/j.1748-7692.2005.tb01256.x
Kennedy, A.S., Zerbini, A.N., Vasquez, O.V., Gandilhon, N., Clapham P.J. and Adam, O. (2013). Local and migratory movements of humpback whales (Megaptera novaeangliae) satellite-tracked in the North Atlantic Ocean. Canadian Journal of Zoology, 92; 8-17. https://doi.org/10.1139/cjz-2013-0161
Laist, D.W., Knowlton, A.R., Mead, J.G., Collet, A.S. and Podesta, M. (2001). Collisions between ships and whales. Marine Mammal Science, 17, 35-75. https://doi.org/10.1111/j.1748-7692.2001.tb00980.x
Larsen, A.H., Øien, N., Vikingsson, G. and Palsboll, P. (1996). Populations genetic analysis of nuclear and mitochondrial loci in skin biopsies collected from central and northeastern North Atlantic humpback whales (Megaptera novaeangliae): Population identity and migratory destinations. Proceedings: Biological Sciences, 263, 1611-1618. https://doi.org/10.1098/rspb.1996.0236
Lawson, J. and Gosselin, J-F. (2018). Estimates of cetacean abundance from the 2016 NAISS aerial surveys of eastern Canadian waters, with a comparison to estimates from the 2007 TNASS. SC/25/AE/09 for the NAMMCO Scientific Committee.
Leonard, D.M. and Øien, N.I. (2020a). Estimated Abundances of Cetacean Species in the Northeast Atlantic from Two Multiyear Surveys Conducted by Norwegian Vessels between 2002–2013. NAMMCO Scientific Publications, 11. https://doi.org/10.7557/3.4695
Leonard, D.M. and Øien, N.I. (2020b). Abundance of cetaceans in the Northeast Atlantic estimated from Norwegian shipboard surveys conducted in 2014-2018. NAMMCO Scientific Publications, 11 https://doi.org/10.7557/3.4695
Lusseau, D., Bejder, L. (2007). The long-term consequences of short-term responses to disturbance experiences from whalewatching impact assessment. Int. J. Comp. Psychol., 20, 228-236. http://researchrepository.murdoch.edu.au/id/eprint/1277
Mackay, M. (2015). Occurrence patterns and social behaviors of humpback whales (Megaptera novaeangliae) wintering off Puerto Rico, USA. Texas A&M University. https://hdl.handle.net/1969.1/155581
Magnúsdóttir, E., Rasmussen, M., Lammers, M. and Svavarsson, J. (2014). Humpback whale songs during winter in subarctic waters. Polar Biology, 37, 427-433. https://doi.org/10.1007/s00300-014-1448-3
Martin, S. (2012). Whale watching in Iceland: An assessment of whale watching activities on Skjalfandi Bay. Master’s Thesis, University of Akureyri, Iceland.
Martin, S. C., Aniceto, A. S., Ahonen, H., Pedersen, G., Lindstrøm, U. (2021). Humpback Whale (Megaptera novaeangliae) Song on a Subarctic Feeding Ground. Frontiers in Marine Science. https://doi.org/10.3389/fmars.2021.669748
McCordic, J.A., Todd, S.K. and Stevick, P.T. (2014). Differential rates of killer whale attacks on humpback whales in the North Atlantic as determined by scarification. Journal of the Marine Biological Association of the United Kingdom, 94, 1311-1315. https://doi.org/10.1017/S0025315413001008
Mikhalev, Y.A. (1997). Humpback whales Megaptera novaeangliae in the Arabian Sea. Mar. Eco. Prog. Ser. 149, 13–21
Mundinger, P. C. (1980). Animal cultures and a general theory of cultural evolution. Sociobiol., 1, 183-223.
Noad, M., Cato, D. H., Bryden, M. M., Jenner, M. N. M., Jenner K. C. S. (2000). Cultural revolution in whale songs. Nature, 408, 537-538. DOI: 10.1038/35046199
North Atlantic Marine Mammal Commission (NAMMCO). (2009). Report of the 16th Scientific Committee Meeting, Reykjavík, Iceland. https://nammco.no/topics/scientific-committee-reports/
North Atlantic Marine Mammal Commission (NAMMCO). (2010). Report of the NAMMCO Expert Group meeting on assessment of large whale killing data. https://nammco.no/hunting-methods-expert-group-reports/
North Atlantic Marine Mammal Commission (NAMMCO). (2011). Report of the 18th Scientific Committee Meeting, Gjógv, Faroe Islands. https://nammco.no/topics/scientific-committee-reports/
North Atlantic Marine Mammal Commission (NAMMCO). (2014). Manual for the maintenance and use of weaponry and equipment deployed in hunting of baleen whales in NAMMCO member countries. Available at https://nammco.no/hunting-manuals/
North Atlantic Marine Mammal Commission (NAMMCO). (2015). Report of the Expert Group Meeting on assessing time to death data from the large whale hunts. Available at https://nammco.no/hunting-methods-expert-group-reports/
North Atlantic Marine Mammal Commission (NAMMCO). (2017). Report of the 24th Scientific Committee Meeting, Reykjavík, Iceland. https://nammco.no/topics/scientific-committee-reports/
North Atlantic Marine Mammal Commission (NAMMCO). (2019). Report of the 26th Scientific Committee Meeting, Tórshavn, Faroe Islands. https://nammco.no/topics/scientific-committee-reports/
O’Connor, S., Campbell, R., Cortez, H. and Knowles, T. (2009). Whale Watching Worldwide: tourism numbers, expenditures and expanding economic benefits, a special report from the International Fund for Animal Welfare, Yarmouth MA, USA, prepared by Economists at Large.
Pallin, L. J., Baker, S., Steel, D., Kellar, N. M., Robbins, J., Johnston, D. W., Nowacek, D. P., Read, A. J., Friedlaender, A. S. (2018). High pregnancy rates in humpback whales (Megaptera novaeangliae) around the Western Antarctic Peninsula, evidence of a rapidly growing population. Royal Society Open Science, 5, 5. https://doi.org/10.1098/rsos.180017
Palka D. (2012). Cetacean abundance estimates in US northwestern Atlantic Ocean waters from summer 2011 line transect survey. US Dept Commer, Northeast Fish Sci Cent Ref Doc. 12-29; 37 p. Available at http://www.nefsc.noaa.gov/nefsc/publications/
Paxton, C.G.M, Burt, M.L., Hedley, S.L., Víkingsson, G.A., Gunnlaugsson, Th. and Desportes, G. (2009). Density surface fitting to estimate the abundance of humpback whales based on the NASS-95 and NASS-2001 aerial and shipboard surveys. NAMMCO Scientific Publications, 7, 143-159. https://doi.org/10.7557/3.2711
Payne, R. S., McVay, S. (1971). Songs of Humpback Whales. Science, 173, 585-597.
Pike, D.G., Paxton, C.G.M., Gunnlaugsson, Th. and Víkingsson, G.A. (2009a). Trends in the distribution and abundance of cetaceans from aerial surveys in Icelandic coastal waters, 1986-2001. NAMMCO Scientific Publications, 7, 117-142. https://doi.org/10.7557/3.2710
Pike, D.G., Gunnlaugsson, T., Mikkelsen, B., Halldórsson, S.D. and Víkingsson, G.A. (2019). Estimates of the abundance of cetaceans in the central North Atlantic based on the NASS Icelandic and Faroese shipboard surveys conducted in 2015. NAMMCO Scientific Publications, 11. https://doi.org/10.7557/3.4941
Pike, D.G., Gunnlaugsson, T., Mikkelsen, B., Halldórsson, S.D., Víkingsson, G.A., Acquarone, M. and Desportes, G. (2020a). Estimates of the abundance of cetaceans from the T-NASS Icelandic and Faroese ship surveys conducted in 2007. NAMMCO Scientific Publications, 11. https://doi.org/10.7557/3.5269
Pike, D.G., Gunnlaugsson, T., Víkingsson, G.A. and Sigurjónsson, J. (2020b). Distribution and abundance of cetaceans in Icelandic waters over 30 years of aerial surveys. NAMMCO Scientific Publications, 11. https://doi.org/10.7557/3.4805
Punt, A.E., Friday, N.A. and Smith, T.D. (2007). Reconciling data on the trends and abundance of North Atlantic humpback whales within a population modelling framework. Journal of Cetacean Research and Management, 8, 145. https://archive.iwc.int/pages/search.php?search=%21collection15&k=
Ramp, C., Delarue, J., Palsbøll, P.J., Sears, R. and Hammond, P.S. (2015). Adapting to a Warmer Ocean—Seasonal Shift of Baleen Whale Movements over Three Decades. Plos ONE, 10, 1-15. https://doi.org/10.1371/journal.pone.0121374
Reilly, S.B., Bannister, J.L., Best, P.B., Brown, M., Brownell Jr., R.L., Butterworth, D.S., Clapham, P.J., Cooke, J., Donovan, G.P., Urbán, J. and Zerbini, A.N. (2008). Megaptera novaeangliae. The IUCN Red List of Threatened Species 2008: e.T13006A3405371. http://dx.doi.org/10.2305/IUCN.UK.2008.RLTS.T13006A3405371.en
Ryan, C., McHugh, B., Boyle, B., McGovern, E., Bérubé, M., Lopez-Suárez, P., … and Clapham, P. J. (2013). Levels of persistent organic pollutants in eastern North Atlantic humpback whales. Endangered Species Research, 22, 213-223. https://doi.org/10.3354/esr00545
Ryan, C., Wenzel, F.W., Lopez Suarez, P. and Berrow, S.D. (2014). An abundance estimate for humpback whales Megaptera novaeangliae breeding around Boa Vista, Cape Verde Islands. Zoologia Caboverdiana, 5, 20-28.
Schuler, A. R., Piwetz, S., Clemente, J. D., Steckler, D., Mueter, F., Pearson, H. C. (2019). Humpback whale movements and behavior response to whale-watching vessels in Juneau, AK. Frontiers in Marine Science. https://doi.org/10.3389/fmars.2019.00710
Silber GK. (1986). The relationship of social vocalizations to surface behavior and aggression in the Hawaiian humpback whale (Megaptera novaeangliae). Can J Zool 64(10):2075–2080. https://doi.org/10.1139/z86-316
Simon, M., Johnson, M. and Madsen, P.T. (2012). Keeping momentum with a mouthful of water: behavior and kinematics of humpback whale lunge feeding. Journal Of Experimental Biology, 215, 3786-3798. https://doi.org/10.1242/jeb.071092
Skern-Mauritzen, M. (2010). Grazing baleen whales in the Barents Sea: Mostly krill or a bit of everything? Fisken og Havet, Special Edition 2-2010. https://www.hi.no/resources/Marine-mammals-.pdf
Smith, T. D., Allen, J., Clapham, P. J., Hammond, P. S., Katona, S., Larsen, F., … and Øien, N. (1999). An ocean‐basin‐wide mark‐recapture study of the North Atlantic humpback whale (Megaptera novaeangliae).Marine Mammal Science, 15, 1-32. https://doi.org/10.1111/j.1748-7692.1999.tb00779.x
Smith, T. D. and Reeves, R. R. (2011). Historical Catches of Humpback Whales, Megaptera novaeangliae, in the North Atlantic Ocean: Estimates of Landings and Removals. Marine Fisheries Review, 73, 1-43.
Smith, T.D. and Pike, D.G. (2009). The enigmatic whale: The North Atlantic humpback. NAMMCO Scientific Publications, 7, 161-178. https://doi.org/10.7557/3.2712
Sprogis, K. R., Videsen, S., Madsen, P. T. (2020) Vessel noise levels drive behavioural responses of humpback whales with implications for whale-watching. eLife. DOI: 10.7554/eLife.56760
Stevick, P.T., Berrow, S.D., Bérubé, M., Bouveret, L., Broms, F., Jann, B., Kennedy, A., López Suárez, P., Meunier, M., Ryan, C. and Wenzel, F. (2016). There and back again: multiple and return exchange of humpback whales between breeding habitats separated by an ocean basin. Journal of the Marine Biological Association of the United Kingdom, 96, 885–890. https://doi.org/10.1017/S0025315416000321
Stimpert, A. K., Wiley, D. N., Au, W. W. L., Johnson, M. P. & Arsenault, R. (2007). ‘Megapclicks’: acoustic click trains and buzzes produced during night-time foraging of humpback whales (Megaptera novaeangliae). Lett.3, 467–70. doi: 10.1098/rsbl.2007.0281
Tyack, P. (1981). Interactions between singing Hawaiian humpback whales and conspecifics nearby. Ecol. Sociobiol., 8, 105-116.
Vigness‐Raposa, K.J., Kenney, R.D., Gonzalez, M.L. and August, P.V. (2010). Spatial patterns of humpback whale (Megaptera novaeangliae) sightings and survey effort: Insight into North Atlantic population structure. Marine Mammal Science, 26, 161-175. https://doi.org/10.1111/j.1748-7692.2009.00336.x
Víkingsson, G.A. (2004). Langreyður (Fin whale). In Páll Hersteinsson (Ed.), Íslensk Spendýr (Icelandic Mammals) (pp. 204-211). Reykjavik: Vaka-Helgafell.
Víkingsson, G.A., Pike, D.G., Valdimarsson, H., Schleimer, A., Gunnlaugsson, T., Silva, T., … and Bogason, V. (2015). Distribution, abundance, and feeding ecology of baleen whales in Icelandic waters: have recent environmental changes had an effect?. Frontiers in Ecology and Evolution, 3, 6. https://doi.org/10.3389/fevo.2015.00006
Weinrich, M. and Corbelli, C. (2009). Does whale watching in southern New England impact humpback whale (megaptera novaeangliae) calf production or calf survival? Biological Conservation, 142, 2931-2940. https://doi.org/10.1016/j.biocon.2009.07.018
Wenzel, F.W., Allen, J., Berrow, S., Hazevoet, C.J., Jann, B., Seton, R.E., … and Whooley, P. (2009). Current knowledge on the distribution and relative abundance of humpback whales (Megaptera novaeangliae) off the Cape Verde Islands, Eastern North Atlantic. Aquatic Mammals, 35, 502-510. https://doi.org/10.1578/AM.35.4.2009.502
Øien, N. (2009). Distribution and abundance of large whales in Norwegian and adjacent waters based on ship surveys 1995 – 2001. NAMMCO Scientific Publications, 7, 31-48. https://doi.org/10.7557/3.2704
General Information pages
Norwegian Polar Institute – Humpback whale
Institute of Marine Research Norway – Knolhval (in Norwegian)
Greenland Institute of Natural Resources – Photo identification of humpback whales
Marine and Freshwater Research Institute of Iceland – Humpback whale photo identification database
Department of Fisheries and Oceans Canada – Humpback whale catalogue
Canadian Species at Risk Public Registry – Humpback whale














